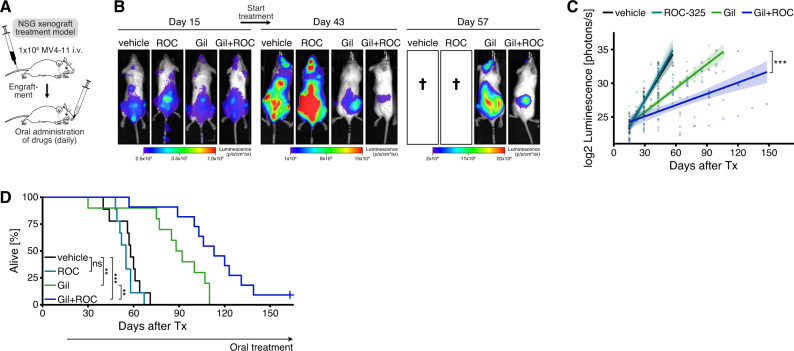
Fig. 5. Pharmacological autophagy inhibition cooperates synergistically with FLT3 inhibition in vivo to increase antileukemic efficacy against xenografted human FLT3-ITD + AML cells in mice.
A In vivo xenograft treatment model, schematic overview. Luciferase-labeled MV4-11 cells were transplanted into NSG (non-obese diabetic (NOD)/severe combined immunodeficient (SCID)/Il2rg−/−) mice by intravenous injection. After engraftment, mice were treated daily with [1] methylcellulose vehicle, [2] ROC-325 (ROC) 50 mg/kg, [3] gilteritinib (gil) 3 mg/kg, or [4] gilteritinib 3 mg/kg + ROC-325 50 mg/kg by oral gavage. B Representative serial bioluminescence images on day 15 after transplantation prior to treatment start and at later time points on-treatment. C Quantification of serial dorsal bioluminescence measurements during treatment. Individual measurements and group-wise log2-linear regressions are shown. Shaded regions indicate 95% confidence intervals of regression lines. P value by linear regression, comparing slope coefficients between gilteritinib and gilteritinib + ROC-325 group (***, P < 0.001). D Kaplan-Meier curves showing overall survival of xenografted NSG mice treated with either vehicle (n = 9), ROC-325 (n = 9), gilteritinib (n = 10), or gilteritinib + ROC-325 (n = 11). P values by robust Cox regression analysis (ns, not significant; **, P < 0.01; ***, P < 0.001). See also Supplementary Fig. 17.