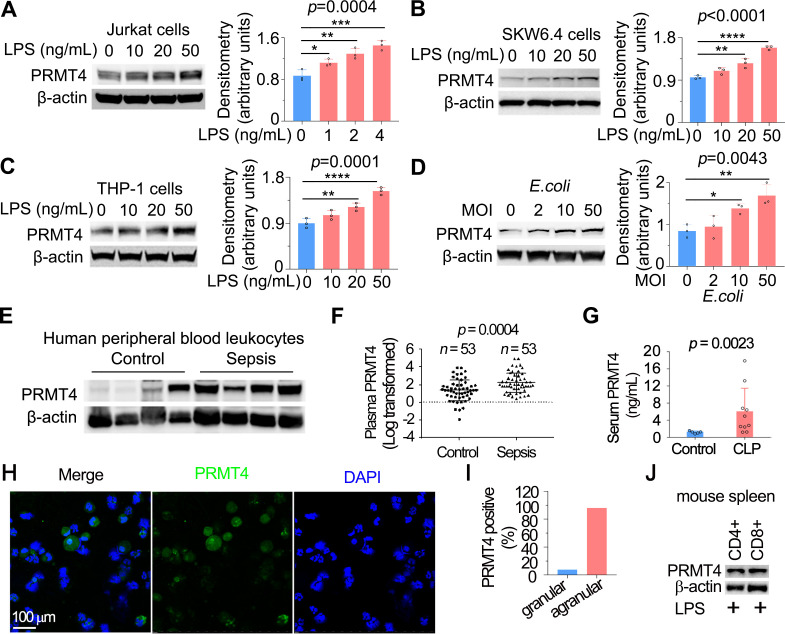
Figure 1.
LPS and Escherichia coli increases PRMT4 protein expression in lymphocytes in vitro, and PRMT4 is increased in experimental septic models. (A–C) Jurkat cells (A), SKW6.4 cells (B) and THP-1 cells (C) were treated with LPS as indicated, and cell lysates were immunoblotted with PRMT4 and β-actin antibodies. Independent experiments, n=3. (D) Jurkat cells were treated with live E. coli as indicated, and cell lysates were immunoblotted with PRMT4 and β-actin antibodies. Densitometry was plotted in the lower panel. Independent experiments, n=3. (E) Lysates of peripheral blood leucocytes from deidentified human samples with or without sepsis were immunoblotting analysed with PRMT4 and β-actin. (F) PRMT4 protein levels were determined by ELISA from blood plasma from septic patients (n=53) and non-septic control patients (n=53). Lines indicate the median and IQR, Mann-Whitney U test, p=0.0004. (G) CLP procedures were subjected to C57BL/6 J mice for 48 hours; mice sera were collected from untreated controls (n=5) and polymicrobial infected mice (n=10) for PRMT4 ELISA analysis. (H, I) Leucocytes isolated from BALF in LPS-treated mouse were immunofluorescent stained with PRMT4 antibody. PRMT4 expression was visualised using confocal microscopy; the nuclei were stained by DAPI (H). Total cells were counted and positively stained granular and agranular cells were presented as percentage (I). A total of 300 granulocytes and 100 agranulocytes were counted. (J) Isolated CD4+ and CD8+ cells from LPS-treated mouse were lysed and immunoblotting analysed with PRMT4 antibody. Independent experiments, n=3. Scale bar=100 µm. *P=0.05–0.01, **P=0.01–0.001, ***P=0.001–0.0001, ****P<0.0001. BALF, bronchoalveolar lavage fluid; CLP, cecal ligation and puncture; DAPI, (4′,6-diamidino-2-phenylindole); LPS, lipopolysaccharide; PRMT4, protein arginine N-methyltransferase 4.