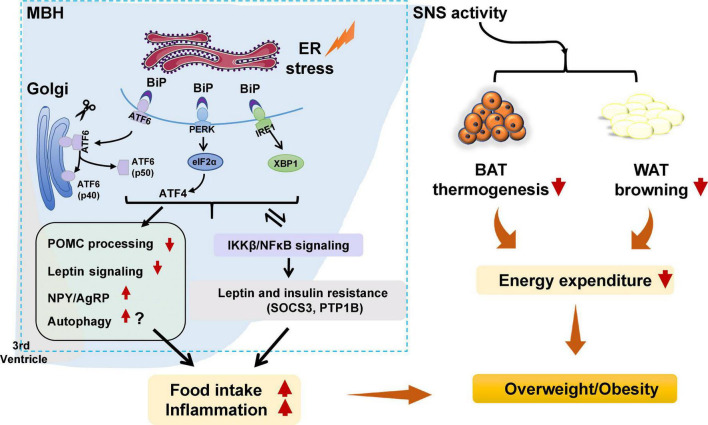
FIGURE 1.
Potential role of hypothalamic endoplasmic reticulum (ER) stress in obesity and inflammation. Under certain stimuli, such as high-fat diet (HFD) feeding, drug treatment, or infection, ER stress is activated, and binding immunoglobulin protein (BiP) dissociates from protein kinase R-like ER kinase (PERK), inositol requiring enzyme 1 (IRE1), and activating transcription factor 6 (ATF6), resulting in the release of those three proteins. PERK is then activated by phosphorylation and p-PERK phosphorylates eukaryotic initiation factor 2α (eIF2α) and increases the translation of ATF4. ATF6 relocates to the Golgi apparatus and is processed by site 1 and 2 proteases, resulting in ATF6 activation. IRE1 is activated by phosphorylation. pIRE1 catalyzes X-box-binding protein 1 (XBP1) mRNA splicing, resulting in increased production of active spliced XBP1. These effects could (1) activate the hypothalamic autophagy signaling, impede proopiomelanocortin (POMC) processing, attenuate leptin signaling, and possibly increase neuropeptide Y (NPY), and agouti-related peptide (AgRP) expression, leading to increased food intake, decreased energy expenditure, inflammation, and weight gain; (2) decrease white adipose tissue (WAT) browning and brown adipose tissue (BAT) thermogenesis by affecting sympathetic nervous system (SNS) activity, resulting in lower energy expenditure and weight gain; and (3) trigger activation of the inhibitor of nuclear factor kappa-B kinase subunit beta (IKKβ)/nuclear factor κB (NF-κB) signaling pathway, resulting in hypothalamic leptin and insulin resistance (by affecting suppressor of cytokine signaling 3 [SOCS3] and protein tyrosine phosphatase 1B [PTP1B]). These effects could increase food intake, decrease energy expenditure, and promote inflammation, thus resulting in weight gain. Furthermore, activated IKKβ/NF-κB signaling could lead to ER stress in the hypothalamus and worsen hypothalamic inflammation.