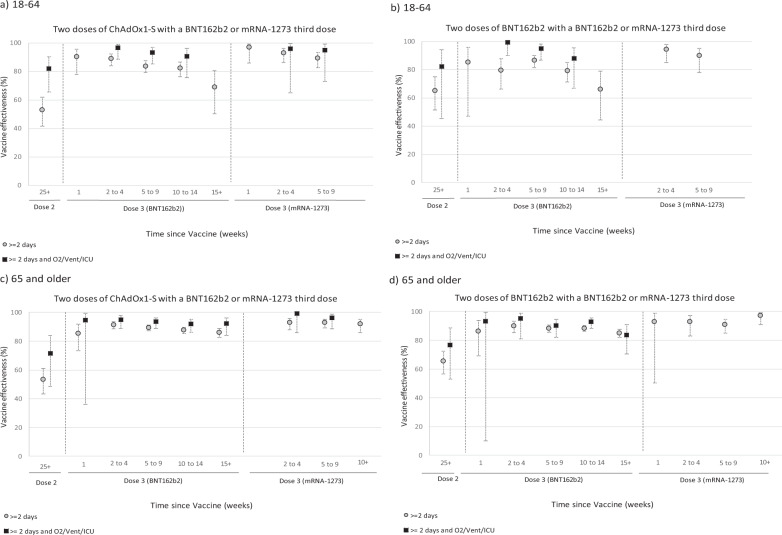
Fig. 3. Vaccine effectiveness estimates with 95% confidence intervals against hospitalisations >=2 days and >=2 days and on oxygen/ventilated/on ICU using SUS by age group and manufacturer (all symptomatic controls, Omicron only).
Vaccine effectiveness estimates are adjusted using sex, index of multiple deprivation (quintile), ethnic group, care home residence status (for age 65+), geographic region (NHS region), period (calendar week of the test), health and social care worker status (for age <65), clinical risk group status (for age <65), clinically extremely vulnerable, severely immunosuppressed, and previously tested positive. a 18–64: two doses of ChAdOx1-S with a BNT162b2 or mRNA-1273 third dose, b 18–64: two doses of BNT162b2 with a BNT162b2 or mRNA-1273 third dose, c 65 and older: two doses of ChAdOx1-S with a BNT162b2 or mRNA-1273 third dose, d 65 and older: two doses of BNT162b2 with a BNT162b2 or mRNA-1273 third dose.