Abstract
BACKGROUND/OBJECTIVES
Previous studies have reported that protein supplementation contributes to the attenuation of inflammation. Serious trauma such as burn injury usually results in the excessive release of inflammatory factors and organs dysfunction. However, a few reports continued to focus on the function of protein ingestion in regulating burn-induced inflammation and organ dysfunction.
MATERIALS/METHODS
This study established the rat model of 30% total body surface area burn injury, and evaluated the function of blended protein (mixture of whey and soybean proteins). Blood routine examination, inflammatory factors, blood biochemistry, and immunohistochemical assays were employed to analyze the samples from different treatment groups.
RESULTS
Our results indicated a decrease in the numbers of white blood cells, monocytes, and neutrophils in the burn injury group administered with the blended protein nutritional support (Burn+BP), as compared to the burn injury group administered normal saline supplementation (Burn+S). Expressions of the pro-inflammatory factors (tumor necrosis factor-α and interleukin-6 [IL-6]) and chemokines (macrophage chemoattractant protein-1, regulated upon activation normal T cell expressed and secreted factor, and C-C motif chemokine 11) were dramatically decreased, whereas anti-inflammatory factors (IL-4, IL-10, and IL-13) were significantly increased in the Burn+BP group. Kidney function related markers blood urea nitrogen and serum creatinine, and the liver function related markers alanine transaminase, aspartate aminotransferase, alkaline phosphatase, and lactate dehydrogenase were remarkably reduced, whereas albumin levels were elevated in the Burn+BP group as compared to levels obtained in the Burn+S group. Furthermore, inflammatory cells infiltration of the kidney and liver was also attenuated after burn injury administered with blended protein supplementation.
CONCLUSIONS
In summary, nutritional support with blended proteins dramatically attenuates the burn-induced inflammatory reaction and protects organ functions. We believe this is a new insight into a potential therapeutic strategy for nutritional support of burn patients.
Keywords: Soy-whey blended protein, ingestion, burns, inflammation, tissues damage
INTRODUCTION
Proteins are essential nutrients for subsistence, which play important roles in body composition, enzyme synthesis, tissue repair, and maintaining immunity functions. The basic units of proteins are amino acids, classified as either essential amino acids or non-essential amino acids, depending on whether the body is able to synthesize them. Essential amino acids required by the human body must be acquired from the diet, and their content and ratio are used to evaluate the quality of protein in food. Whey protein is isolated from milk and is easy to digest and absorb. It contains all the essential amino acids, and the ratio of which is close to the human body’s requirements. Soybean protein isolated from soybeans also contains all the essential amino acids (except methionine, which is present in relatively low levels). Whey and soybean protein are generally considered high-quality proteins obtained from animals and plants, respectively. They are widely used as protein nutritional supplements for the elderly or patients afflicted with wasting diseases.
Protein nutritional support has numerous benefits for health and contributes to patient recovery [1]. Protein supplementation was expected to attenuate skeletal muscle atrophy, and animal model experiments and clinical trials both verified that dietary supplementation of protein significantly attenuates muscle weight loss, improves muscle function, and reduces aging or immobilization-induced muscle atrophy [2,3]. The classical mechanisms include inactivating the ubiquitin-proteasome system, reducing oxidative response, and suppressing inflammatory reaction; moreover, potential new mechanisms such as altering the miRNA expression and microbiota abundance were also detected [4,5,6]. Previous studies indicate that whey protein or soybean protein treatment regulates the expression of inflammation related factors and mitigates the inflammatory reaction [7,8]. Blended protein supplementation was also reported to be beneficial in mitigating exercise-induced fatigue, which is probably the outcome of blended protein ingestion increasing the skeletal muscle cell transporter expression and promoting amino acid transport after exercise [9,10]. Blended protein was also easy for obtaining a more balanced amino acid profile; the contents of leucine, arginine, and methionine were higher than amounts obtained from a single protein source [11]. Clinical trials further revealed that blended protein intake promotes skeletal muscle protein synthesis, which helps improve the muscle condition of hematopoietic stem cell posttransplant patients with leukemia [12,13]. In general, protein ingestion played an important role in reducing inflammation, balancing protein metabolism, and maintaining skeletal muscle function. However, blended protein has the potential to exert more effective functions, which has placed great expectations on clinical nutritional intervention.
Burn injuries are usually caused by heat, electricity, chemicals, and other factors, and occur more frequently in developing countries. Excessive inflammatory reaction and multiple organ dysfunction syndrome (MODS) are key factors restricting the clinical therapy of burn patients. Due to destruction of the skin integrity, pathogen invasion induces activation of immune cells which secrete excessive inflammatory factors and triggers immune dysfunction. Long-time immune dysfunction might further result in the occurrence of sepsis, leading to MODS and even death [14]. Reducing excessive inflammation is one of the important ways to promote burn wound healing. Our previous studies indicate that the human umbilical cord mesenchymal stem cells derived exosome treatment significantly down-regulates the burn-induced inflammatory reaction [15], and stem cells transplantation noticeably accelerates wound healing [16]. Moreover, soybean peptides application is also reported to effectively regulate the secretion of inflammatory factors and promote burn wound healing [17]. However, there is little publication focusing on the effect of protein nutritional support on organ protection after burn injury.
In the current study, whey protein and soybean protein were blended and used for protein nutritional support by gavage, and effects on regulating burn-induced inflammatory reaction and organ function were observed. Our results indicate that blended protein treatment effectively alleviates the burn-induced inflammatory cell increment, reduces the expressions of pro-inflammatory factors, elevates the anti-inflammatory factor levels, attenuates inflammatory cells infiltration, and protects organ functions. In summary, blended protein application is a potentially novel method for the clinical nutritional strategy of burn patients to help attenuate the inflammatory reaction and protect organ functions.
MATERIALS AND METHODS
Materials
Whey protein isolate and soybean protein isolate were purchased from Hilmar Ingredients (Livingston, CA, USA) and Shaanxi Pioneer Biotech Co. Ltd. (Xi’an, Shaanxi, China), respectively. Normal saline was procured from Shijiazhuang No.4 Pharmaceutical Co. Ltd. (Shijiazhuang, Hebei, China). The antibodies against CD68 (ab125212) and MPO (ab208670) were purchased from Abcam (Cambridge, MA, USA).
Animal model
Male SD rats (6-wk-old, 180–220 g weight) were housed at room temperature (22–24°C) with a 12 h day-night cycle. All studies adhered to procedures consistent with the International Guiding Principles for Biomedical Research Involving Animals issued by the Council for the International Organizations of Medical Sciences (CIOMS) and were approved by the Animal Care and Use Committee of the Fourth Medical Center of PLA General Hospital. After adaptive feeding for 1 wk, the rats were anesthetized by intraperitoneal injection of 3% pentobarbital sodium salt. A full thickness burn model with 30% total body surface area (TBSA) was conducted as previously described [18,19]. Briefly, an electric hair cutter was used to shave the dorsal hair. The burn rat model was established by placing the back skin in hot water (94°C) for 12 s; rats in the sham group were placed in warm water (37°C) for 12 s. The wounds were swabbed with 1% tincture of iodine to prevent infection. Fluid infusion was administered via an immediate intraperitoneal injection of balanced salt solution (40 mg/kg·body weight) for the prevention of shock.
Group and treatment
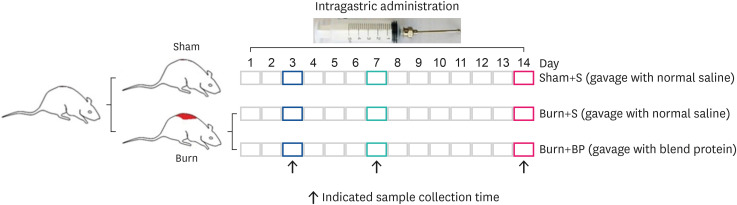
After successful modeling, all rats were administered by gavage once a day for 3, 7 or 14 days. Equivalent whey protein and soybean protein (whey:soybean = 1:1) were directly dissolved in normal saline. Burn rats were randomly divided into 2 groups: (1) Burn+BP, burn rats were administered 2 mL blended protein dissolved in normal saline (200 mg/kg • body weight) by gavage, as described previously [6]; (2) Burn+S (burn rats) and Sham+S (sham rats) were administered 2 ml normal saline by gavage. After the respective treatment of rats in the three treatment groups, the samples were collected at indicated times (Fig. 1).
Fig. 1. Animal model and treatment.
SD rats were used to establish models of sham and burn, as described in “MATERIALS AND METHODS”. Burn rats were randomly divided into Burn+S and Burn+BP groups. Rats in the Sham+S, Burn+S, and Burn+BP groups were administrated the following treatments by gavage: 2 mL normal saline, 2 mL normal saline, and 2 mL blended protein solved in normal saline (200 mg/kg • body weight with 50% whey protein and 50% soybean protein), respectively. Finally, on days 3, 7 and 14, the rats were anesthetized and samples were collected.
Sample collection
At the indicated times, the rats were anesthetized, and blood samples were collected from the abdominal aorta using the procoagulant blood vessel collection (yellow) tube and the anticoagulant blood vessel collection (purple) tube. Liver and kidney tissue was also collected and placed in paraformaldehyde for tissue fixation (Servicebio, Wuhan, Hubei, China).
Blood routine examination
Whole blood was collected using anticoagulant blood vessel collection, and routine blood examination was performed on the Sysmex K4500 hematology analyzer (Kobe, Japan).
Blood biochemistry
Blood samples were collected from the treated animals using procoagulant blood vessel collection tubes, and various indices, including liver function and renal function, were measured by the KHB-ZY1280 (Shanghai Kehua Bio-Engineering Co. Ltd., Shanghai, China).
Immunohistochemical assay
After treatment for 14 days, the liver and kidney tissue samples were harvested from the three treatment groups and subsequently fixed in 4% paraformaldehyde for 24 h at room temperature. Samples were then embedded in paraffin and sliced into 5 mm thick perpendicular plane sections, and immunohistochemical analysis was done following the manufacturer’s instruction kit from Servicebio. The antibodies against CD-68 and MPO, and the HRP labeled corresponding secondary antibody were applied. Treated sections were finally stained by exposure to 3,3’-diaminobenzidine (DAB), and the images were microscopically captured.
Cytokine analysis
Rat Chemokine/Cytokine Panel 22plex (eBioscience, Vienna, Austria) was used to measure the concentration of plasma cytokines, as described in our previous publication [17]. Levels of interleukin (IL)-4, IL-6, IL-10, IL-13, macrophage chemoattractant protein-1 (MCP-1), Eotaxin, and regulated upon activation normal T cell expressed and secreted factor (RANTES) were analyzed using the Luminex™ 200 (Luminex, Shanghai, China).
Statistical analysis
All data are expressed as the mean ± SD and the differences are analyzed using the analysis of variance assay. The difference is considered to be statistically significant at P ≤ 0.05.
RESULTS
Blended protein ingestion ameliorates the burn-induced increment of inflammatory cells
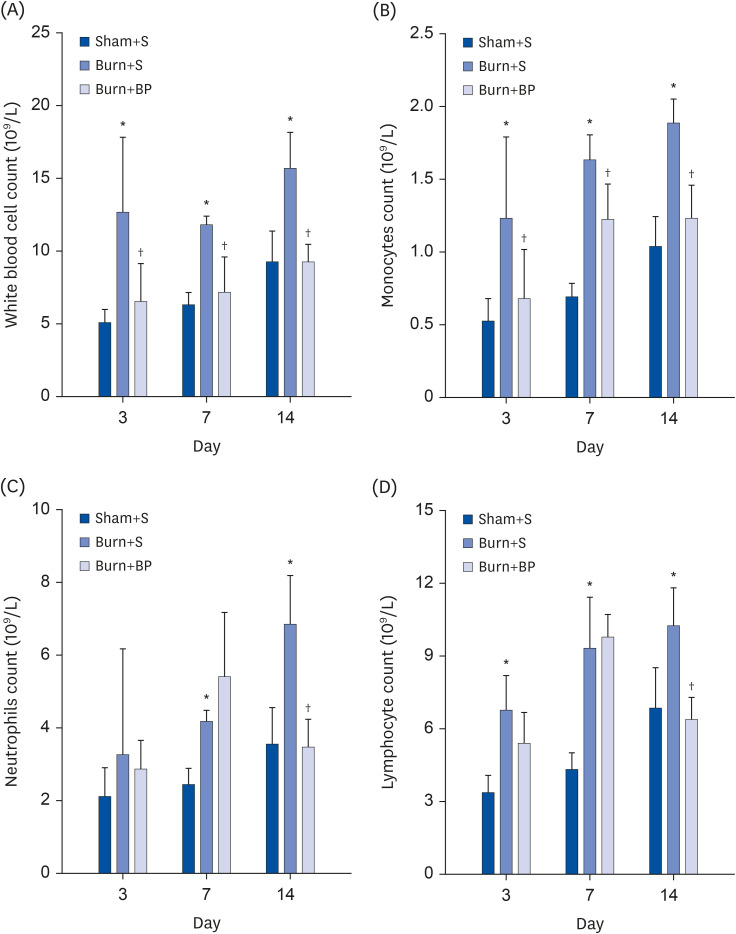
During an inflammatory reaction, there is a dramatic increase in the number of immune related cells, especially white blood cells. This is because the first line of defense in our body, viz., skin integrity, is broken after burn injury, leading to a pathogen invasion and subsequent excessive increment of white blood cells [17]. Therefore, the white blood cell count is one of the primary indicators for evaluating inflammation [20]. Effective treatment for reducing the white blood cell number might help ease the inflammatory reaction. Results of the current study revealed that the number of white blood cells significantly increases on days 3, 7, and 14 after burn injury (Fig. 2A), indicating that an inflammatory reaction is significantly promoted after burn injury. Previous studies have reported that protein nutritional support plays an important role in reducing inflammatory reactions [4,21]. In the current research, blended protein as described in “MATERIALS AND METHODS” was used to treat the burned rats. Compared with rats in the Burn+S group, blended protein supplementation remarkably alleviated the increment of white blood cells on days 3, 7, and 14 (Fig. 2A), thereby indicating that blended protein treatment has the potential to reduce burn-induced inflammation. White blood cells consist of monocytes, granulocytes (neutrophils, eosinophils, and basophils), and lymphocytes. The number of monocytes and neutrophils in different groups was measured, and similar to the variation tendency of white blood cells, the number of monocytes also increased dramatically after burn injury, and significantly reduced with blended protein administration (Fig. 2B). Burn injury also evoked the increment of neutrophils on days 3, 7, and 14. However, the number of neutrophils in the Burn+S group was comparable with those in the Burn+BP group on days 3 and 7, which subsequently decreased dramatically on day 14 (Fig. 2C). The data of the lymphocyte count indicates that burn injury results in a dramatic increase in the number of lymphocytes on days 3, 7, and 14, but blended protein treatment significantly reduces the lymphocyte number on day 14, as compared to numbers obtained in the Burn+S group (Fig. 2D). Taken together, our results indicate that blended protein ingestion might be beneficial in reducing burn-induced increment of inflammation related cells with simultaneous attenuation of the inflammatory reaction.
Fig. 2. Inflammatory cell count using routine blood examination.
Whole blood samples from rats in the Sham+S, Burn+S, and Burn+BP groups were collected and used for routine blood examination. White blood cell count (A), monocytes count (B), neutrophils count (C), and lymphocyte count (D) were analyzed.
*Represents significant difference between the Sham+S and Burn+S groups; †Represents significant difference in the Burn+BP group compared to the Burn+S group. The difference is considered to be statistically significant at P ≤ 0.05.
Blended protein supplementation regulates the burn-induced expression of cytokines
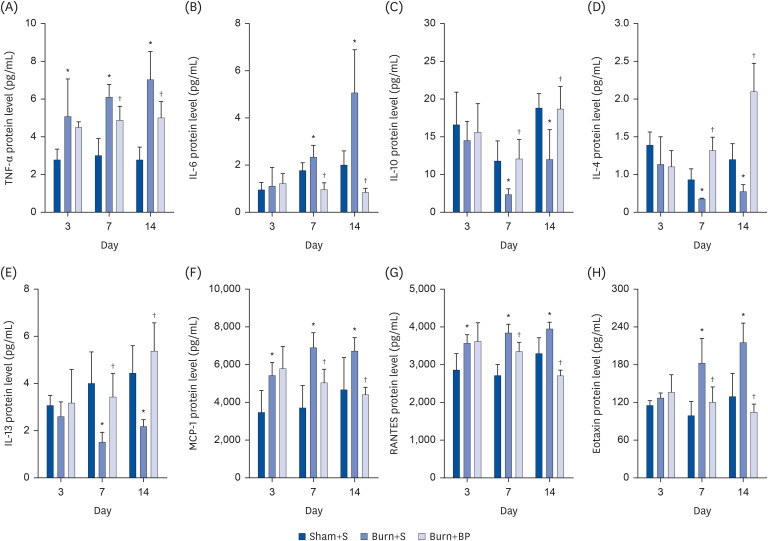
Cytokines are usually secreted by inflammation related cells such as monocytes, lymphocytes, and neutrophils, and exert important roles in regulating the inflammatory reaction. Subsequent to a burn injury, pro-inflammatory factors such as tumor necrosis factor-α (TNF-α) and IL-6 are usually overexpressed, and the level of anti-inflammatory factor IL-10 is down-regulated [15,16,17]. Chemokines play important roles in recruiting inflammatory cells and promoting cell infiltration to the inflammatory lesions [22]. In order to detect the influence of blended protein administration on the expression of cytokines after burn injury, we measured the levels of pro-inflammatory factors, anti-inflammatory factors, and chemokines, using the Rat Chemokine/Cytokine Panel 22plex kit. As shown in Figure 3, the expression of pro-inflammatory factors TNF-α and IL-6 were significantly increased after burn injury, compared to levels obtained in the Sham+S group, on days 3, 7, and 14; moreover, the blended protein ingestion significantly down-regulated the burn-induced TNF-α and IL-6 overexpression on days 7 and 14 (Fig. 3A and B). Compared to the Sham+S group, expression of the anti-inflammatory factor IL-10 was dramatically decreased after burn injury on days 7 and 14, which recovered after the blended protein treatment (Fig. 3C). IL-4 and IL-13 are other important anti-inflammatory factors and attenuate the inflammatory reaction via regulation of macrophage polarization [23]. Consistent with the tendency of IL-10, secretion of IL-4 and IL-13 was also obviously suppressed after burn injury on days 7 and 14, but significantly increased in the blended protein supplementation group (Fig. 3D and E). These results indicate that a burn injury results in elevating the expressions of pro-inflammatory factors (TNF-α and IL-6) and reducing the levels of anti-inflammatory factors (IL-4, IL-10, and IL-13), but administration of blended protein remarkably reverses this phenomenon. This might be a partial mechanism of blended protein attenuating burn-induced inflammation. Administration of blended protein also exerted an effect on regulating chemokine expression. MCP-1/chemokine (C-C motif) ligand 2 (CCL2) regulates the release of monocytes from the bone marrow and mediates migration to the inflamed sites [24]. RANTES/CCL5 enhances chemotaxis of immune cells and is involved in trafficking them to the inflammatory lesions [25]. Eotaxin, a potent chemoattractant of eosinophils, stimulates eosinophils migration [26]. Hence, all chemokines effectively regulate the inflammatory reaction via mediating chemotaxis of the inflammatory cells. Our results indicate that burn injury promotes the expressions of MCP-1, RANTES, and Eotaxin on days 3, 7, and 14, but blended protein ingestion significantly reduces the expressions on days 7 and 14 (Fig. 3F-H), thereby exhibiting the potential role of blended protein treatment in reducing burn-induced inflammatory cell infiltration. Taken together, our results indicate the beneficial role of blended protein nutritional support in alleviating an inflammatory reaction and reducing inflammatory cells infiltration, thereby playing an essential role in protecting organ function after burn injury.
Fig. 3. Expression of cytokines in rats with various treatments.
After different treatments, the serum was collected at indicated times, and the cytokine level was measured using the Rat Chemokine/Cytokine Panel 22plex kit, following the manufacturer’s instructions. Pro-inflammatory factors [TNF-α (A) and IL-6 (B)], anti-inflammatory factors [IL-10 (C), IL-4 (D), and IL-13 (E)], and chemokines [MCP-1 (F), RANTES (G), and Eotaxin (H)] were analyzed in the Sham+S, Burn+S, and Burn+BP groups.
*Represents significant difference between the Sham+S and Burn+S groups; †Represents significant difference in the Burn+BP group compared to the Burn+S group. The difference is considered to be statistically significant at P ≤ 0.05.
Blended protein diet attenuates burn-induced inflammatory cells infiltration
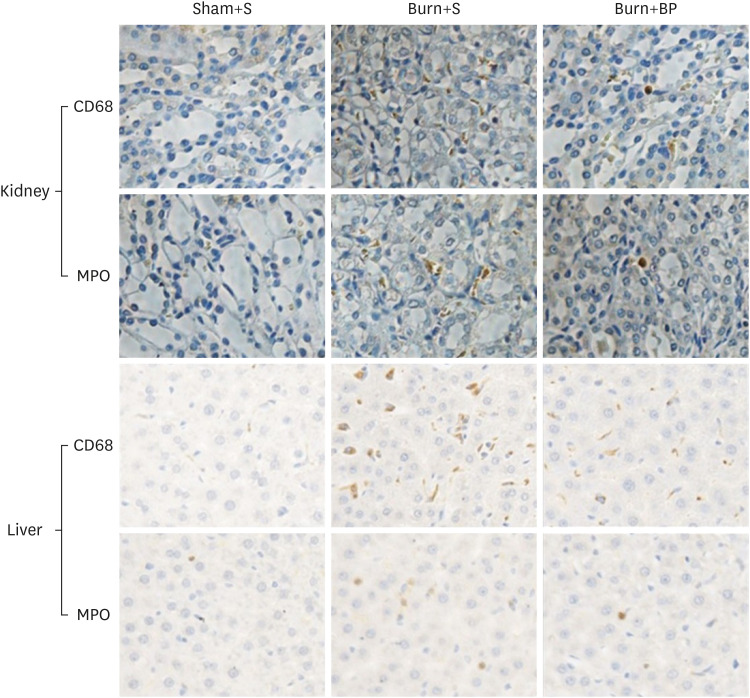
Organ dysfunction is a common adverse effect observed in patients with burn injury. Kidney and liver functions are usually impaired after major burn injuries, partially due to the excessive inflammatory reaction and inflammatory cells infiltration [27,28]. To determine the function of blended protein in regulating inflammatory cells infiltration, we evaluated the expressions of CD68 (macrophage marker) and MPO (neutrophil marker) by subjecting the kidney and liver tissues to immunohistochemical assays. As presented in Fig. 4, the significantly increased expressions of CD68 and MPO in the kidney tissue after burn injury were effectively reduced after blended protein supplementation, in comparison to the expressions determined in the Burn+S group (upper panel). This suggests that blended protein treatment contributes to reducing the macrophage and neutrophil cells infiltration of kidney tissue. Similar results were obtained in the liver tissue; macrophage and neutrophil cells infiltration was promoted after burn injury, but was remarkably inhibited after blended protein supplementation (Fig. 4, lower panel). Previous publications have reported that attenuating macrophage and neutrophil cells infiltration results in ameliorating kidney and liver damage [29,30]. Therefore, inhibiting inflammatory cells infiltration might be a key strategy for protecting the liver and kidney functions after burn injury. In summary, blended protein ingestion exerts an important role in reversing burn-induced increment of inflammatory cells infiltration, and potentially attenuates damage to the kidney and liver.
Fig. 4. Inflammatory cells infiltration in kidney and liver tissues.
After treatment for 14 days, the kidney and liver tissues of rats in all 3 groups were harvested and fixed in 4% paraformaldehyde for at least 24 h at room temperature. Fixed samples were then embedded in paraffin and sliced into 5 mm thick sections. Antibodies against CD68 (surface biomarker of macrophage) and MPO (surface biomarker of neutrophils) were used to reflect macrophage and neutrophils infiltration in the kidney and liver tissues. The yellow color represents a positive staining cell (400×).
MPO, myeloperoxidase.
Blended protein nutriment improves kidney function
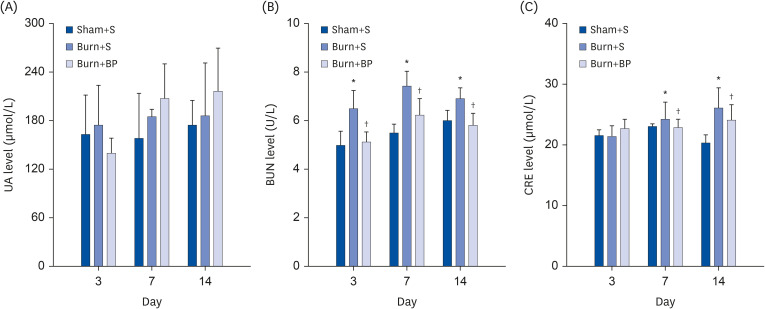
To further analyze the kidney function of rats in different groups, the levels of serum uric acid (UA), blood urea nitrogen (BUN), and creatinine (CRE) were measured. It has previously been demonstrated that consuming a high protein diet risks increasing the kidney burden and elevates serum UA levels [31,32]. Assessing the UA level is generally applied as an early clue for judging kidney injury [33]. Especially in burn patients, gastrointestinal congestion leads to impaired functions of digestion and absorption, which further augments the concern of protein nutrition increasing kidney burden [34]. However, results from a clinical trial indicate that isolated soybean protein with flaxseed oil or corn oil nutritional support has no significant effect on increasing the kidney burden [35]. In the current study, we observed slightly increased UA levels after burn injury on days 3, 7, and 14, which were further elevated on days 7 and 14 after blended protein administration, but with no significant difference observed between the three groups (Fig. 5A). This indicates that a blended protein diet has no risk of increasing the kidney burden, which is probably because whey and soybean proteins are known to be easily digested and absorbed [36]. Previous studies also reported that soybean protein consumption slows the decline of the estimated glomerular filtration rate and significantly improves proteinuria in patients with nephropathy [37]. Other parameters such as BUN and CRE, which reflect the renal filtration function, are critical indicators of kidney function. Our results demonstrate that compared to the Burn+S group, burn injury led to a remarkable increment of BUN levels on days 3, 7, and 14, with noticeably reduced levels obtained after blended protein administration (Fig. 5B). Although the CRE level was comparable in all three groups on day 3, a similar tendency with BUN levels was observed on days 7 and 14, being dramatically up-regulated in the Burn+S group and down-regulated in the Burn+BP group (Fig. 5C). These results indicate that burn injury elevates the levels of BUN and CRE, implying the possibility of kidney function impairment. However, blended protein consumption effectively reduces the levels of BUN and CRE after burn injury, and might exert an essential role in protecting kidney function.
Fig. 5. Blended protein ingestion regulates kidney function related indices.
After the indicated treatment, the kidney function related indices were measured. (A) UA level showed no significant difference among the three groups. (B) BUN level dramatically increased after burn injury, and was reversed with blended protein ingestion on days 3, 7 and 14. (C) CRE level was comparable among the 3 group on day 3, then up-regulated after burn injury, and down-regulated in the Burn+BP group on days 7 and 14.
UA, uric acid; BUN, blood urea nitrogen; CRE, creatinine.
*Represents significant difference between the Sham+S and Burn+S groups; †Represents significant difference in the Burn+BP group compared to the Burn+S group. The difference is considered to be statistically significant at P ≤ 0.05.
Blended protein administration improves liver function
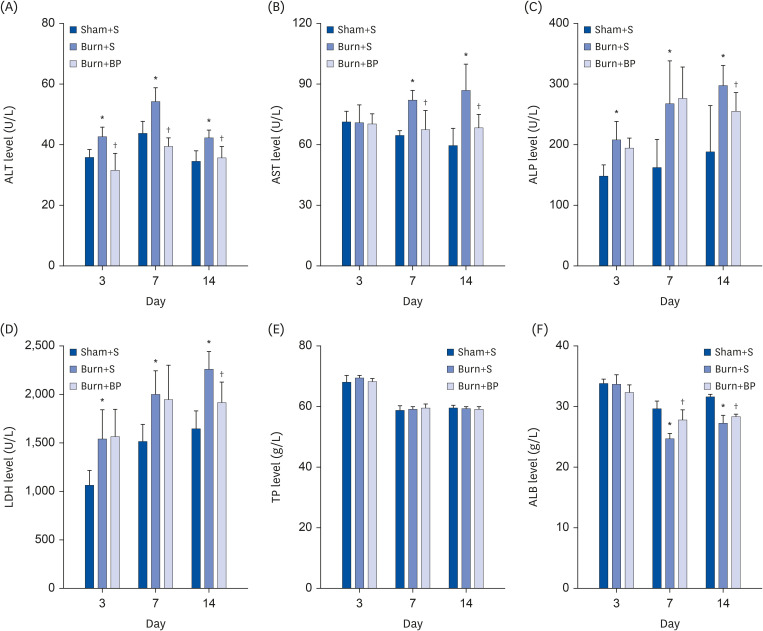
The liver is also one of the organs liable to be damaged after burn injury. In order to evaluate liver function, related indices were evaluated, including alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), and lactate dehydrogenase (LDH). As shown in Fig. 6A, a significant increase was observed in the ALT level after burn injury on days 3, 7, and 14 compared to the Sham+S group, which were dramatically down-regulated after blended protein treatment. The level of AST was comparable among the three groups on day 3, but was obviously elevated after burn injury on days 7 and 14, and dramatically down-regulated in the Burn+BP group (Fig. 6B). Similar results were obtained for ALP and LDH levels; burn injury led to significant increment of ALP and LDH levels compared to the Sham+S group on days 3, 7, and 14. However, blended protein ingestion remarkably reduced the ALP and LDH expressions on day 14 (Fig. 6C and D). ALT and AST are mainly synthesized by liver cells, and their high levels in serum imply the occurrence of liver injury [38]. ALP and LDH are widely distributed in the liver, skeletal muscle, and kidney, and are therefore also important indices to determine liver injury [39]. Our results indicate that burn injury induces liver injury, while blended protein supplementation contributes to improving the liver function. Biosynthesis is one of the important functions of the liver, and is an essential site for protein synthesis in our body. Thus, the levels of total protein (TP) and albumin (ALB) are also important for evaluating liver function. The serum levels of TP and ALB were therefore measured in all three groups. Our results show no significant change in the total protein level among the three groups on days 3, 7, and 14 (Fig. 6E). Although the level of ALB was comparable among the 3 groups on day 3, a dramatic reduction of ALB level was observed after burn injury on days 7 and 14 (Fig. 6F), implying that ALB synthesis is suppressed after burn injury. Compared to the value obtained in the Burn+S group, the ALB level was remarkably restored after blended protein administration (Fig. 6F), indicating that blended protein nutritional intervention helps to improve the liver capacity for ALB synthesis. Taken together, all the results (including ALT, AST, ALP, LDH, and ALB) provide compelling evidence that blended protein consumption diminishes burn-induced liver injury and exerts an essential role in liver protection.
Fig. 6. Blended protein ingestion regulates liver function related indices.
The liver function related indices were determined after different treatments. Levels of ALT (A), AST (B), ALP (C), LDH (D), TP (E), and ALB (F) were further analyzed in the Sham+S, Burn+S, and Burn+BP groups.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; LDH, lactate dehydrogenase; TP, total protein; ALB, albumin.
*Represents significant difference between the Sham+S and Burn+S groups; †Represents significant difference in the Burn+BP group compared to the Burn+S group. The difference is considered to be statistically significant at P ≤ 0.05.
DISCUSSION
Excessive inflammatory reaction is a major adverse effect after burn injury. Our results validated the upregulation of pro-inflammatory factors (TNF-α and IL-6) and downregulation of anti-inflammatory factors (IL-4, IL-10, and IL-13) after a burn injury. However, blended protein ingestion significantly suppressed the pro-inflammatory factors and elevated the anti-inflammatory factors. A previous study also indicated that treatment with soybean peptides significantly reduces the inflammatory response and promotes burn wound healing [17]. The hypermetabolic state, which can usually can persist for years after recovery and is a common phenomenon of burn patients [40], was partially evoked by the systemic inflammatory reaction [41,42]. Subsequent studies confirmed that supplementation with soybean peptides ameliorates muscle loss after burn injury, and blended protein treatment also results in ameliorating load swimming-induced fatigue and enhancing muscle function [10,19]. A clinical study also showed that blended protein nutritional support improves the skeletal muscle outcomes of transplantation patients with hematological malignancies [13]. Results from these studies, therefore, imply that protein nutrient supplementation has the potential for attenuating burn-induced hypermetabolism. Hypermetabolic activation not only results in increased catabolism and a tremendous loss of lean body mass, but also leads to a decline of immune function. Thus, one of the recommendations from clinical studies for nutritional support of burn patients suggests increasing protein consumption [43,44]. In the current study, the ALB level (which is closely related to immune function) was significantly decreased in the Burn+S group but restored after blended protein treatment. This indicates that protein consumption is potentially beneficial in reducing burn-induced catabolism and maintaining the immune function.
Damage to the liver and kidney are commonly known to occur during MODS, which is one of the major lethal factors for burn patients [45]. Inflammatory cells infiltration increases after burn injury, which might result in enhancing acute injury of the organs [46,47]. Our results indicate that dietary nutrition with blended protein not only reduces the burn-induced inflammatory reaction, but also inhibits infiltration of inflammatory cells to the liver and kidney tissues (Fig. 4). This research validates the considerable potential of blended protein ingestion in protecting organ functions after a burn injury.
In summary, these encouraging results indicate that blended protein nutrient supplementation reduces the burn-induced systemic inflammatory responses, attenuates inflammatory cells infiltration, and improves kidney and liver functions. To the best of our knowledge, this study is the first to demonstrate that blended protein ingestion is implicated in ameliorating burn-induced inflammatory cells infiltration and protecting liver and kidney functions. This study provides a novel and attractive potential nutritional intervention for burn patients to ameliorate an inflammatory reaction and protect organ functions.
Footnotes
Funding: This work was supported by the Beijing Nova Program (Z181100006218043), the Beijing Technology and Innovation Plan (Z191100009318015), the Innovation Engineering Project of the Chinese Academy of Agricultural Sciences, the Beijing Natural Science Foundation (7172210), and the National Natural Science Foundation of China (NSFC81471873).
Conflict of Interest: The authors declare no potential conflicts of interests.
- Conceptualization: Yu Y, Zhang J, Wang J.2
- Formal analysis: Yu Y, Zhang J, Wang J1, Chai J.
- Investigation: Zhang J, Yu Y.
- Writing - original draft: Yu Y, Zhang J.
- Writing - review & editing: Yu Y, Wang J.2
1Wang J, Jing Wang (Beijing Technology and Business University); 2Wang J, Jing Wang (Ministry of Agriculture and Rural Affairs).
References
- 1.Yu Y, Gaine GK, Zhou L, Zhang J, Wang J, Sun B. The classical and potential novel healthy functions of rice bran protein and its hydrolysates. Crit Rev Food Sci Nutr. 2021 doi: 10.1080/10408398.2021.1929057. [DOI] [PubMed] [Google Scholar]
- 2.Liberman K, Njemini R, Luiking Y, Forti LN, Verlaan S, Bauer JM, Memelink R, Brandt K, Donini LM, Maggio M, et al. Thirteen weeks of supplementation of vitamin D and leucine-enriched whey protein nutritional supplement attenuates chronic low-grade inflammation in sarcopenic older adults: the PROVIDE study. Aging Clin Exp Res. 2019;31:845–854. doi: 10.1007/s40520-019-01208-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shin JE, Park SJ, Ahn SI, Choung SY. Soluble whey protein hydrolysate ameliorates muscle atrophy induced by immobilization via regulating the PI3K/Akt pathway in C57BL/6 mice. Nutrients. 2020;12:3362. doi: 10.3390/nu12113362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang J, Yu Y, Wang J. Protein nutritional support: the classical and potential new mechanisms in the prevention and therapy of sarcopenia. J Agric Food Chem. 2020;68:4098–4108. doi: 10.1021/acs.jafc.0c00688. [DOI] [PubMed] [Google Scholar]
- 5.Yu Y, Zhang J, Wang J, Sun B. MicroRNAs: the novel mediators for nutrient-modulating biological functions. Trends Food Sci Technol. 2021;114:167–175. [Google Scholar]
- 6.Yu Y, Zhou L, Li X, Liu J, Li H, Gong L, Zhang J, Wang J, Sun B. The progress of nomenclature, structure, metabolism, and bioactivities of oat novel phytochemical: avenanthramides. J Agric Food Chem. 2022;70:446–457. doi: 10.1021/acs.jafc.1c05704. [DOI] [PubMed] [Google Scholar]
- 7.Bitzer ZT, Wopperer AL, Chrisfield BJ, Tao L, Cooper TK, Vanamala J, Elias RJ, Hayes JE, Lambert JD. Soy protein concentrate mitigates markers of colonic inflammation and loss of gut barrier function in vitro and in vivo . J Nutr Biochem. 2017;40:201–208. doi: 10.1016/j.jnutbio.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 8.Da Silva MS, Bigo C, Barbier O, Rudkowska I. Whey protein hydrolysate and branched-chain amino acids downregulate inflammation-related genes in vascular endothelial cells. Nutr Res. 2017;38:43–51. doi: 10.1016/j.nutres.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 9.Reidy PT, Walker DK, Dickinson JM, Gundermann DM, Drummond MJ, Timmerman KL, Cope MB, Mukherjea R, Jennings K, Volpi E, et al. Soy-dairy protein blend and whey protein ingestion after resistance exercise increases amino acid transport and transporter expression in human skeletal muscle. J Appl Physiol (1985) 2014;116:1353–1364. doi: 10.1152/japplphysiol.01093.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ren G, Yi S, Zhang H, Wang J. Ingestion of soy-whey blended protein augments sports performance and ameliorates exercise-induced fatigue in a rat exercise model. Food Funct. 2017;8:670–679. doi: 10.1039/c6fo01692h. [DOI] [PubMed] [Google Scholar]
- 11.Liu J, Klebach M, Visser M, Hofman Z. Amino acid availability of a dairy and vegetable protein blend compared to single casein, whey, soy, and pea proteins: a double-blind, cross-over trial. Nutrients. 2019;11:2613. doi: 10.3390/nu11112613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Butteiger DN, Cope M, Liu P, Mukherjea R, Volpi E, Rasmussen BB, Krul ES. A soy, whey and caseinate blend extends postprandial skeletal muscle protein synthesis in rats. Clin Nutr. 2013;32:585–591. doi: 10.1016/j.clnu.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ren G, Zhang J, Li M, Tang Z, Yang Z, Cheng G, Wang J. Gut microbiota composition influences outcomes of skeletal muscle nutritional intervention via blended protein supplementation in posttransplant patients with hematological malignancies. Clin Nutr. 2021;40:94–102. doi: 10.1016/j.clnu.2020.04.030. [DOI] [PubMed] [Google Scholar]
- 14.Yu Y, Chai J, Zhang H, Chu W, Liu L, Ma L, Duan H, Li B, Li D. miR-194 Promotes burn-induced hyperglycemia via attenuating IGF-IR expression. Shock. 2014;42:578–584. doi: 10.1097/SHK.0000000000000258. [DOI] [PubMed] [Google Scholar]
- 15.Li X, Liu L, Yang J, Yu Y, Chai J, Wang L, Ma L, Yin H. Exosome derived from human umbilical cord mesenchymal stem cell mediates MiR-181c attenuating burn-induced excessive inflammation. EBioMedicine. 2016;8:72–82. doi: 10.1016/j.ebiom.2016.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu L, Yu Y, Hou Y, Chai J, Duan H, Chu W, Zhang H, Hu Q, Du J. Human umbilical cord mesenchymal stem cells transplantation promotes cutaneous wound healing of severe burned rats. PLoS One. 2014;9:e88348. doi: 10.1371/journal.pone.0088348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao F, Liu W, Yu YH, Liu XQ, Yin HN, Liu LY, Yi GF. Effect of small molecular weight soybean protein-derived peptide supplementation on attenuating burn injury-induced inflammation and accelerating wound healing in a rat model. RSC Advances. 2019;9:1247–1259. doi: 10.1039/c8ra09036j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu Y, Yang L, Han S, Wu Y, Liu L, Chang Y, Wang X, Chai J. MIR-190B alleviates cell autophagy and burn-induced skeletal muscle wasting via modulating PHLPP1/Akt/FoxO3A signaling pathway. Shock. 2019;52:513–521. doi: 10.1097/SHK.0000000000001284. [DOI] [PubMed] [Google Scholar]
- 19.Zhao F, Yu Y, Liu W, Zhang J, Liu X, Liu L, Yin H. Small Molecular weight soybean protein-derived peptides nutriment attenuates rat burn injury-induced muscle atrophy by modulation of ubiquitin-proteasome system and autophagy signaling pathway. J Agric Food Chem. 2018;66:2724–2734. doi: 10.1021/acs.jafc.7b05387. [DOI] [PubMed] [Google Scholar]
- 20.Pecht T, Gutman-Tirosh A, Bashan N, Rudich A. Peripheral blood leucocyte subclasses as potential biomarkers of adipose tissue inflammation and obesity subphenotypes in humans. Obes Rev. 2014;15:322–337. doi: 10.1111/obr.12133. [DOI] [PubMed] [Google Scholar]
- 21.Rondanelli M, Klersy C, Terracol G, Talluri J, Maugeri R, Guido D, Faliva MA, Solerte BS, Fioravanti M, Lukaski H, et al. Whey protein, amino acids, and vitamin D supplementation with physical activity increases fat-free mass and strength, functionality, and quality of life and decreases inflammation in sarcopenic elderly. Am J Clin Nutr. 2016;103:830–840. doi: 10.3945/ajcn.115.113357. [DOI] [PubMed] [Google Scholar]
- 22.Sakuma M, Khan MA, Yasuhara S, Martyn JA, Palaniyar N. Mechanism of pulmonary immunosuppression: extrapulmonary burn injury suppresses bacterial endotoxin-induced pulmonary neutrophil recruitment and neutrophil extracellular trap (NET) formation. FASEB J. 2019;33:13602–13616. doi: 10.1096/fj.201901098R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Z, Bozec A, Ramming A, Schett G. Anti-inflammatory and immune-regulatory cytokines in rheumatoid arthritis. Nat Rev Rheumatol. 2019;15:9–17. doi: 10.1038/s41584-018-0109-2. [DOI] [PubMed] [Google Scholar]
- 24.Haller H, Bertram A, Nadrowitz F, Menne J. Monocyte chemoattractant protein-1 and the kidney. Curr Opin Nephrol Hypertens. 2016;25:42–49. doi: 10.1097/MNH.0000000000000186. [DOI] [PubMed] [Google Scholar]
- 25.Fichna M, Żurawek M, Budny B, Komarowska H, Niechciał E, Fichna P, Ruchała M. Elevated serum RANTES chemokine levels in autoimmune Addison disease. Pol Arch Intern Med. 2018;128:216–221. doi: 10.20452/pamw.4221. [DOI] [PubMed] [Google Scholar]
- 26.Rankin SM, Conroy DM, Williams TJ. Eotaxin and eosinophil recruitment: implications for human disease. Mol Med Today. 2000;6:20–27. doi: 10.1016/s1357-4310(99)01635-4. [DOI] [PubMed] [Google Scholar]
- 27.Yagi H, Soto-Gutierrez A, Kitagawa Y, Tilles AW, Tompkins RG, Yarmush ML. Bone marrow mesenchymal stromal cells attenuate organ injury induced by LPS and burn. Cell Transplant. 2010;19:823–830. doi: 10.3727/096368910X508942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inoue Y, Yu YM, Kurihara T, Vasilyev A, Ibrahim A, Oklu R, Zhao G, Nair AV, Brown D, Fischman AJ, et al. Kidney and liver injuries after major burns in rats are prevented by resolvin D2. Crit Care Med. 2016;44:e241–e252. doi: 10.1097/CCM.0000000000001397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaneko Y, Cho T, Sato Y, Goto K, Yamamoto S, Goto S, Madaio MP, Narita I. Attenuated macrophage infiltration in glomeruli of aged mice resulting in ameliorated kidney injury in nephrotoxic serum nephritis. J Gerontol A Biol Sci Med Sci. 2018;73:1178–1186. doi: 10.1093/gerona/gly019. [DOI] [PubMed] [Google Scholar]
- 30.Lamas-Paz A, Hao F, Nelson LJ, Vázquez MT, Canals S, Gómez Del Moral M, Martínez-Naves E, Nevzorova YA, Cubero FJ. Alcoholic liver disease: utility of animal models. World J Gastroenterol. 2018;24:5063–5075. doi: 10.3748/wjg.v24.i45.5063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kołodziej U, Maciejczyk M, Niklińska W, Waszkiel D, Żendzian-Piotrowska M, Żukowski P, Zalewska A. Chronic high-protein diet induces oxidative stress and alters the salivary gland function in rats. Arch Oral Biol. 2017;84:6–12. doi: 10.1016/j.archoralbio.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 32.Machín M, Simoyi MF, Blemings KP, Klandorf H. Increased dietary protein elevates plasma uric acid and is associated with decreased oxidative stress in rapidly-growing broilers. Comp Biochem Physiol B Biochem Mol Biol. 2004;137:383–390. doi: 10.1016/j.cbpc.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 33.Pan J, Shi M, Ma L, Fu P. Mechanistic insights of soluble uric acid-related kidney disease. Curr Med Chem. 2020;27:5056–5066. doi: 10.2174/0929867326666181211094421. [DOI] [PubMed] [Google Scholar]
- 34.Ng JW, Cairns SA, O’Boyle CP. Management of the lower gastrointestinal system in burn: a comprehensive review. Burns. 2016;42:728–737. doi: 10.1016/j.burns.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 35.Babajafari S, Hojhabrimanesh A, Sohrabi Z, Ayaz M, Noorafshan A, Akrami A. Comparing isolated soy protein with flaxseed oil vs isolated soy protein with corn oil and wheat flour with corn oil consumption on muscle catabolism, liver function, blood lipid, and sugar in burn patients: a randomized clinical trial. Trials. 2018;19:308. doi: 10.1186/s13063-018-2693-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nelms CL. Optimizing enteral nutrition for growth in pediatric chronic kidney disease (CKD) Front Pediatr. 2018;6:214. doi: 10.3389/fped.2018.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McGraw NJ, Krul ES, Grunz-Borgmann E, Parrish AR. Soy-based renoprotection. World J Nephrol. 2016;5:233–257. doi: 10.5527/wjn.v5.i3.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li M, Zhao H, Wu J, Wang L, Wang J, Lv K, Liu S, Wang M, Guan W, Liu J, et al. Nobiletin protects against acute liver injury via targeting c-Jun N-terminal kinase (JNK)-induced apoptosis of hepatocytes. J Agric Food Chem. 2020;68:7112–7120. doi: 10.1021/acs.jafc.0c01722. [DOI] [PubMed] [Google Scholar]
- 39.Qiao M, Yang J, Zhu Y, Zhao Y, Hu J. Transcriptomics and proteomics analysis of system-level mechanisms in the liver of apigenin-treated fibrotic rats. Life Sci. 2020;248:117475. doi: 10.1016/j.lfs.2020.117475. [DOI] [PubMed] [Google Scholar]
- 40.Epting CL, McBride ME, Wald EL, Costello JM. Pathophysiology of post-operative low cardiac output syndrome. Curr Vasc Pharmacol. 2016;14:14–23. doi: 10.2174/1570161113666151014123718. [DOI] [PubMed] [Google Scholar]
- 41.Clark A, Imran J, Madni T, Wolf SE. Nutrition and metabolism in burn patients. Burns Trauma. 2017;5:11. doi: 10.1186/s41038-017-0076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klein GL. The role of the musculoskeletal system in post-burn hypermetabolism. Metabolism. 2019;97:81–86. doi: 10.1016/j.metabol.2019.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rousseau AF, Losser MR, Ichai C, Berger MM. ESPEN endorsed recommendations: nutritional therapy in major burns. Clin Nutr. 2013;32:497–502. doi: 10.1016/j.clnu.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 44.Moore FA, Phillips SM, McClain CJ, Patel JJ, Martindale RG. Nutrition support for persistent inflammation, immunosuppression, and catabolism syndrome. Nutr Clin Pract. 2017;32:121S–127S. doi: 10.1177/0884533616687502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lami FH, Al Naser RK. Epidemiological characteristics of burn injuries in Iraq: a burn hospital-based study. Burns. 2019;45:479–483. doi: 10.1016/j.burns.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 46.Korkmaz HI, Ulrich MM, van Wieringen WN, Vlig M, Emmens RW, Meyer KW, Sinnige P, Krijnen PA, van Zuijlen PP, Niessen HW. The local and systemic inflammatory response in a pig burn wound model with a pivotal role for complement. J Burn Care Res. 2017;38:e796–e806. doi: 10.1097/BCR.0000000000000486. [DOI] [PubMed] [Google Scholar]
- 47.Stoppe C, Averdunk L, Goetzenich A, Soppert J, Marlier A, Kraemer S, Vieten J, Coburn M, Kowark A, Kim BS, et al. The protective role of macrophage migration inhibitory factor in acute kidney injury after cardiac surgery. Sci Transl Med. 2018;10:eaan4886. doi: 10.1126/scitranslmed.aan4886. [DOI] [PubMed] [Google Scholar]