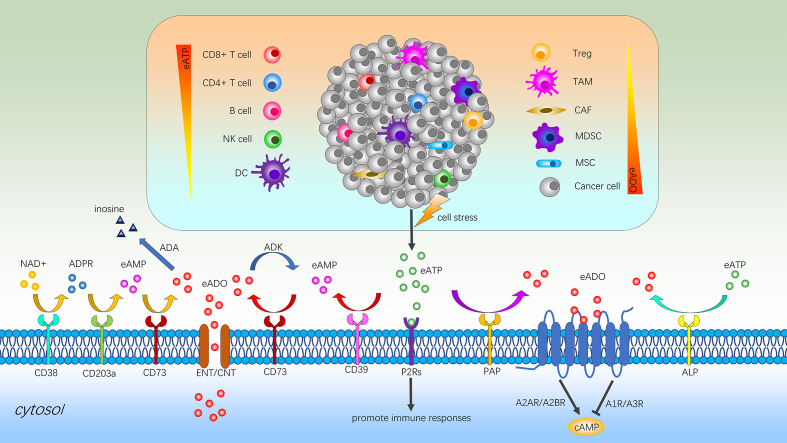
Figure 4.
Immune regulation of adenosine signaling in the TME. Cell stress promotes eATP production and contributes to chronic inflammation via P2Rs. Within the TME, accumulated eATP can be degraded to ADO by the sequential action of the ectonucleotidases CD39 and CD73 or other alternative pathways such as ALP or PAP-mediated process. In addition, the sequential catabolism of NAD+ by CD38, CD203a and CD73 also can generate ADO and the high concentration of intracellular ADO can be transported outside the cell via ENTs or CNTs to maintain balance. The bioavailability of extracellular ADO is regulated by adenosine-converting enzymes such as ADK and ADA, which converts ADO into AMP and inosine respectively. High concentrations of ADO binding to adenosine receptors to inhibit the activation of immune cells and stimulate immunosuppressive cells to promote the immune escape of cancers. eATP, extracellular adenosine triphosphate; eAMP, extracellular adenosine monophosphate; NK cell, natural killer cell; DC, dendritic cell; Treg, regulatory T cell; TAM, tumor-associated macrophage; CAF, cancer associated fibroblast; MDSC, myeloid-derived suppressor cell; MSC, mesenchymal stromal cell; ADO, adenosine; NAD+, nicotinamide adenine dinucleotide; ADPR, adenosine diphosphate ribose; ADA, adenosine deaminase; ADK, adenosine kinase; ENT, equilibrative nucleoside transporter; CNT, concentrative nucleoside transporter; P2Rs, P2 purinergic receptors; PAP, prostatic acid phosphatase; ALP, alkaline phosphatase; cAMP, cyclic adenosine monophosphate.