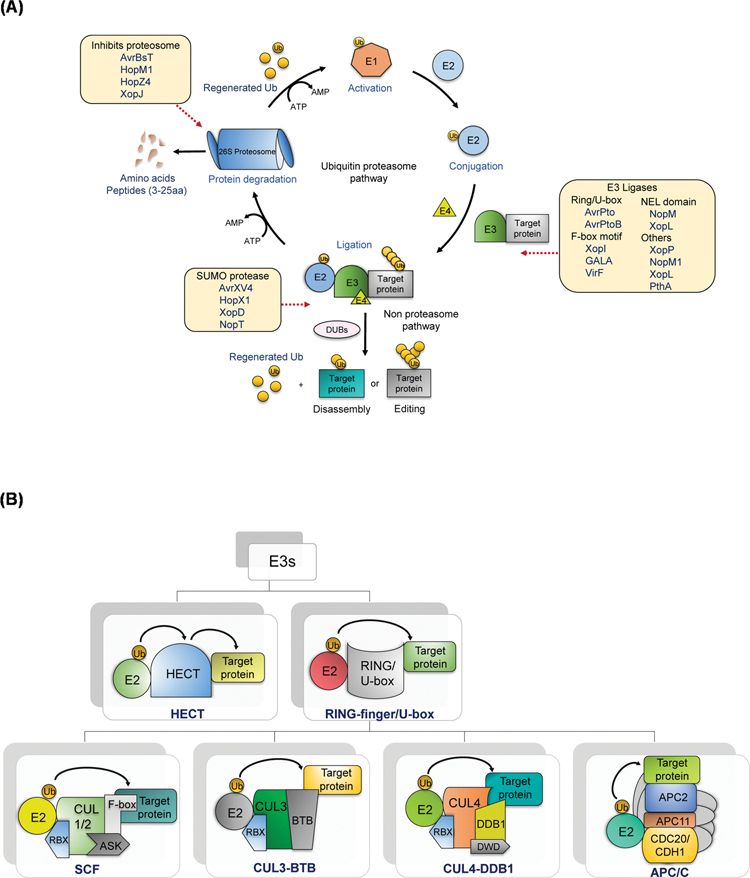
Fig. 2.
(A). Ubiquitination-26S proteasome pathway.
Ubiquitin is first activated by ubiquitin-activating enzyme (E1), at the expenditure of ATP. Then, the ubiquitin molecule is passed on to the second enzyme, ubiquitin-conjugating enzyme (E2). The final enzyme, ubiquitin ligase (E3), recognizes target substrate and binds and labels it with the ubiquitin. The poly-ubiquitination is facilitated by E4, which transfers additional ubiquitin moieties. Proteins modified by sequential linkage of multiple ubiquitin residues of at least four via ubiquitin degradation are targeted by the 26S proteasome. In the non-proteasomal pathway, deubiquitinase (DUBs) catalyze the disassembly and editing of the Ub moieties attached to protein substrates. The various plant effectors that interfere with the UPS are indicated along the pathway.
(B). Plant E3 ubiquitin ligases.
E3 can be divided into HECT and RING/U-box domain-containing E3s based on their mode of transfer of Ub. RING E3s catalyze the transfer of Ub, whereas in the HECT, the E3 forms an intermediate and transfers to the target. The RING/U-box E3s are the multi-subunit complex of E3s viz., SCF, CUL3-BTB, CUL4- DDB1 and APC/C.