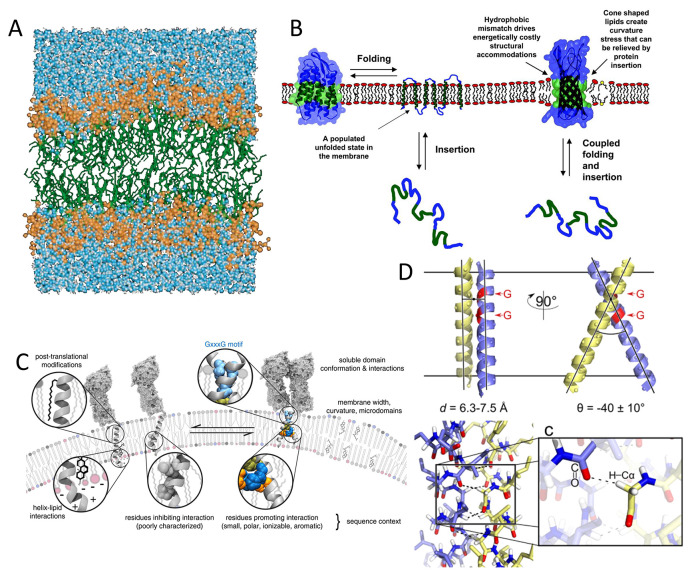
Figure 14.
(A) MD simulation of a hydrated lipid bilayer, including a hydrocarbon layer ∼30 Å and two headgroup layers ∼15 Å each. Reprinted with permission from ref (209). Copyright 2000 Annual Reviews. (B) Schematic for membrane protein folding. For α-helical proteins, helix insertion and packing can be separated; stable transmembrane helices can present without tertiary structure. For β-barrel proteins, folding and insertion are likely to be coupled. Mismatch between the hydrophobic width of the protein (green region) and the bilayer induces distortions in either the protein or the bilayer. Reprinted with permission from ref (311). Copyright 2004 National Academy of Sciences. (C) Factors affecting the affinity of transmembrane helices with putative “dimerization motifs” such as GX3G. A generic transmembrane dimer model is used for illustration, based on a chimera of BNIP3 transmembrane dimer and QSOX (quiescin/sulfhydryl oxidase) soluble domain. Reprinted with permission from ref (339). Copyright 2015 American Chemical Society. (D) Structure for GASright motif of a right-handed helical dimer and a crossing angle of approximately −40°. GX3G sequence enables backbone contact at the crossing point (red), and formation of interhelical H bonds between Cα-H donors and carbonyl oxygen acceptors. Reprinted with permission from ref (347). Copyright 2017 American Chemical Society.