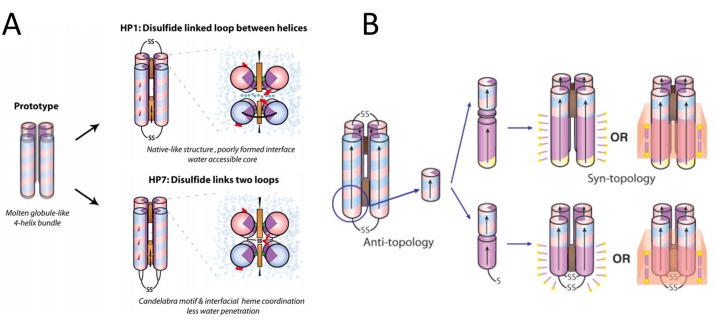
Figure 20.
(A) Schematic representations of HP1 and HP7 where the hydrophobic interior is purple, the alternating positive and negative charges on the protein surface are shown as blue and pink, and glutamate residues are red. HP1 is oriented in an antitopology with a poorly defined intermonomer interface that allows water access into the core. The candelabra motif of HP7 prohibits water access to the core through the use of hemes and a disulfide bond to fix the intermonomer interface. Reprinted with permission from ref (510). Copyright 2008 Portland Press. (B) Schematic of the AP family design and assembly. Positive residues are colored blue, negative is red, polar uncharged are yellow, and nonpolar residues are purple. The incorporation of heme is a brown box. The HP1 sourced HP domain was linked to an LP domain by a flexible linker (AP1, top) or directly (AP3, bottom). AP maquettes can readily assemble with detergents to form micelles or with lipids to form membranes. Reprinted with permission from ref (512). Copyright 2005 American Chemical Society.