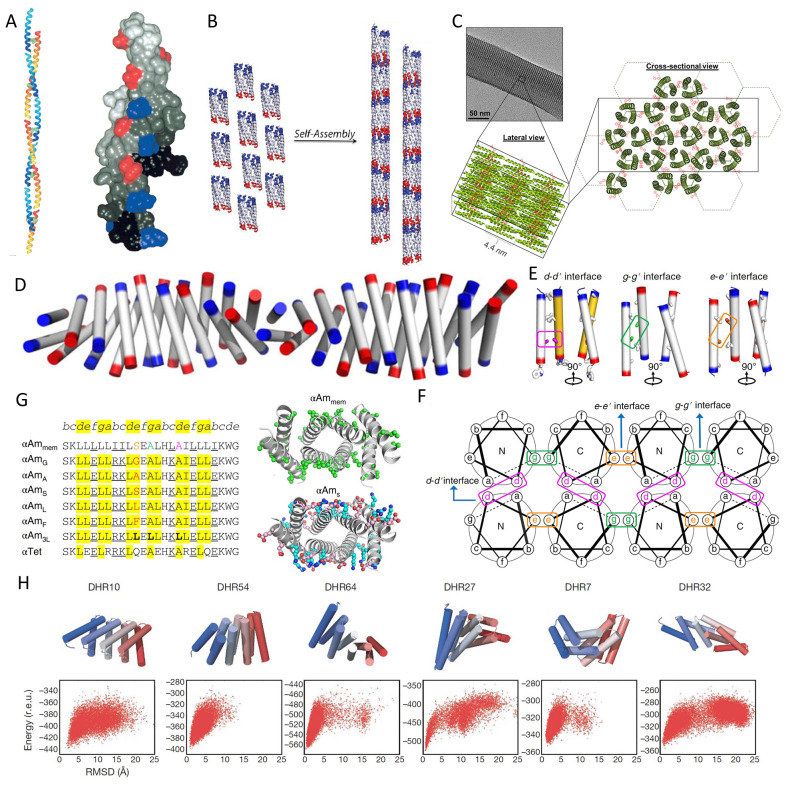
Figure 24.
(A) Computer modeling of the designed self-assembling fiber SAF-p1 (colored yellow-to-red from the N- to the C-terminus) and SAF-p2 (colored blue-to-cyan from the N- to the C-terminus) to form two strands of a staggered, parallel, coiled-coil fiber (left). Negatively charged E (red) and positively charged K (blue) residues form complementary charge interactions (right). Reprinted with permission from ref (551). Copyright 2000 American Chemical Society. (B) Lock-washer structure derived from the GCN4-based seven-helix bundle (PDB ID: 2HY6) in helical nanotubes. Blue and red surfaces represent N- and C-terminus heptads at the interfaces. Reprinted with permission from ref (164). Copyright 2013 American Chemical Society. (C) TEM image with inset depicting the coiled-coil trimer packing within the microstructure, and cross-sectional view of hexagonal close-packing. Reprinted with permission from ref (553). Copyright 2018 American Chemical Society. (D–G) Design of soluble cross-α amyloid fibrils. Reprinted with permission from ref (162). Copyright 2018 Zhang et al. (D) Structure of α-amyloid assembly αAmS (PDB ID: 6C4Z). The N- and C-termini are colored in blue and red, respectively. (E) Three types of helix–helix interfaces with small-residue packing exist in the αAmmem (PDB ID: 6C4X). A17 (magenta), A13 (green), and S11 (orange) are involved in the interhelical packing with larger hydrophobic residues (white sticks) occurring at positions filling the space as helices diverge from the point of closest approach near small residues. (F) Illustration of interhelical d–d′, g–g′, and e–e′ interfaces for the amyloid-like structure with a parallel dimer as the subunit. The small residues and the corresponding interfaces in the helix wheels are boxed. (G) Designed sequences for water-soluble amyloid-like structures compared to αAmmem and sequence changes between the crystal structures of αAmmem (top) versus αAmS (bottom) in the ball-and-stick representation. Hydrophobic residues on the surface of αAmmem are colored green, while the designed residues at the same locations of αAmS are colored cyan and pink for positively and negatively charged, respectively. The mutation on A11 at e–e′ interface is varied to examine its size effect, as shown in red in αAmG (PDB ID: 6C4Y), αAmA, αAmS, αAmL (PDB ID: 6C51), and αAmF. The synergistic effects of varying three small residues to L are tested by αAm3L. A nonaggregating water-soluble αTet (PDB ID: 6C52) is also designed. (H) Six representative designs of repeat protein assembly with design models (top) and computed energy landscapes (bottom). All six landscapes are strongly funneled into the designed energy minimum. Reprinted with permission from ref (559). Copyright 2011 Huang et al.