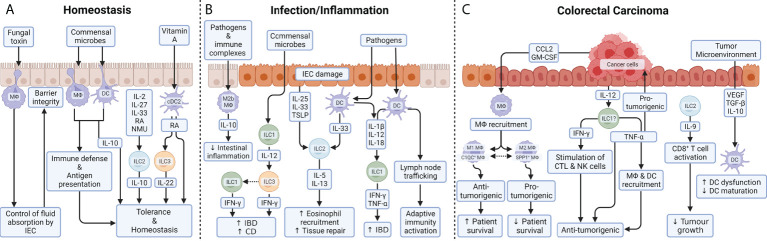
Figure 2.
Myeloid and innate lymphoid cell function in the colon. (A) During homeostasis, balloon-like protrusions formed by LP-MФs sense and limit absorption of fungal toxins by IECs and thus control epithelial integrity. As a large proportion of the luminal microbes are commensals, MФs and DCs must be tolerogenic to prevent inflammation in homeostatic conditions. Their production of IL-10 ensures low responsiveness to stimulation through TLR and balances T cell activity during homeostasis. Under steady-state conditions, a small proportion of intestinal ILC2s also express the anti-inflammatory cytokine IL-10 upon exposure to a variety of exogenous stimuli including IL-2, IL-4, IL-27, IL-10 or neuromedin U (NMU). While the role of ILC2-derived IL-10 remains unclear, it could help to maintain the intestinal mucosa in an anti-inflammatory state. DCs are able to metabolize vitamin A into RA in the intestine. ILC3 can respond to RA, thanks to the transcription factor retinoic acid receptor (RAR) which stimulates IL-22 production under homeostasis as well as during colitis. (B) During inflammatory diseases, an imbalance in the composition of ILC subsets is commonly observed and is thought to contribute to pathogenesis. IL-12 signaling (released by ILC1) gives ILC3 cells the ability to produce IFN-γ. Phenotypic conversion of ILC3 to IFN-γ producing ILC1 is prominent in patients with CD, supporting the pathologic role for IFN-γ secreted from either ILC1 or ILC3. Tissue-resident ILC2s respond to tissue damage and a variety of pathogen-associated danger signals through the expression of receptors for alarmins, such as IL-25R, IL-33R, and thymic stromal lymphopoietin (TSLP) receptor. IL-5 and IL-13 mediate the recruitment of eosinophils and promote tissue repair. After exposure to microbial metabolites, DCs produce IL-12, IL-18 and IL-1β, which are the dominant cytokines required for the induction of IFN-γ and TNF-α by ILC1 during gut inflammation. In response to TLR ligands and immune complexes, a subset of MФs, called regulatory M2b MФs, produce high levels of IL-10 which help reducing intestinal integrity during inflammation. (C) In colorectal carcinomas, cancer cells produce GM-CSF and CCL2 which trigger the recruitment of MФs into the tumor microenvironment. In tumor stroma, a high M1/M2 density ratio was associated with better cancer-specific survival, while preclinical models suggest that genetic or pharmacologic suppression of the M1 to M2 endotype transition reduces colon cancer. In parallel, two populations of MФs, that do not strictly correspond to the M1 or M2 macrophages, have been identified in CRC patients: C1QC+ MФs and SPP1+ MФs. Complement C1q C chain positive MФs (C1QC+ MФs are enriched for complement activation and antigen processing/presentation pathways, indicating their role in anti-tumor responses; whereas secreted phosphoprotein positive MФs 1 SPP1+ MФs express genes involved in tumor angiogenesis and tumor vasculature, suggesting that they play a pro-tumorigenic and pro-metastatic role in CRC. While TNF-α is widely reported to be pro-tumorigenic, the release of IFN-γ and TNF-α by ILCs are believed to be anti-tumorigenic: IFN-γ through stimulation of cytotoxic T cells (CTL) and NK cells and TNF-α through direct induction of apoptosis in tumor cells and tumor vasculature and indirectly through mobilization of MФs and DCs. While ILC1s promote chronic intestinal inflammation via the production of IFN-γ and TNF-α, those same cells are a potential candidates ()? to therapeutically induce IFN-γ and TNF-α for tumor suppression. ILC2s are abundant in colon cancer tissue and are the dominant source of IL-9 which can activate CD8+ T cells to inhibit tumor growth. Colon cancer antigens can induce DCs recruitment, maturation, and cytokine release in order to generate effective TH1-type immune responses. Immunosuppressive signals released by tumor cells or immunomodulatory cells, such as TGF-β, VEGF or IL-10 induce DC dysfunctionality, by inhibiting their production of pro-inflammatory cytokines, and/or prevent DC maturation.