Abstract
To ensure proper immune function, most leukocytes constantly move within tissues or between them using the blood and lymphatic vessels as transport routes. While afferent lymphatic vessels transfer leukocytes from peripheral tissues to draining lymph nodes (dLNs), efferent lymphatics return lymphocytes from LNs back into the blood vascular circulation. Over the last decades, great progress has been made in our understanding of leukocyte migration into and within the lymphatic compartment, leading to the approval of new drugs targeting this process. In this review, we first introduce the anatomy of the lymphatic vasculature and the main cell types migrating through lymphatics. We primarily focus on dendritic cells (DCs) and T cells, the most prominent lymph-borne cell types, and discuss the functional significance as well as the main molecules and steps involved in their migration. Additionally, we provide an overview of the different techniques used to study lymphatic trafficking.
The lymphatic system is composed of primary lymphoid organs (i.e., bone marrow and thymus), secondary lymphoid organs (SLOs), such as spleen and lymph nodes (LNs), and the lymphatic vasculature. The latter consists of a network of lymphatic vessels (LVs), which are present in most vascularized tissues of the body (Petrova and Koh 2018, 2020; Wong et al. 2018). In contrast to the closed blood vascular circulation, the lymphatic vasculature is an open-ended vascular system, which starts at the level of initial afferent lymphatics in peripheral tissues (Fig. 1A; Jackson 2019; Schineis et al. 2019). The main function of initial afferent lymphatics is to drain excess fluid that has leaked out of blood vessels and to return it to the blood circulation. At the same time, initial afferent lymphatics serve as conduits for the trafficking of antigen and leukocytes from peripheral tissues to draining LNs (dLNs), where immune activation, modulation, and induction of tolerance take place (Randolph et al. 2017).
Figure 1.
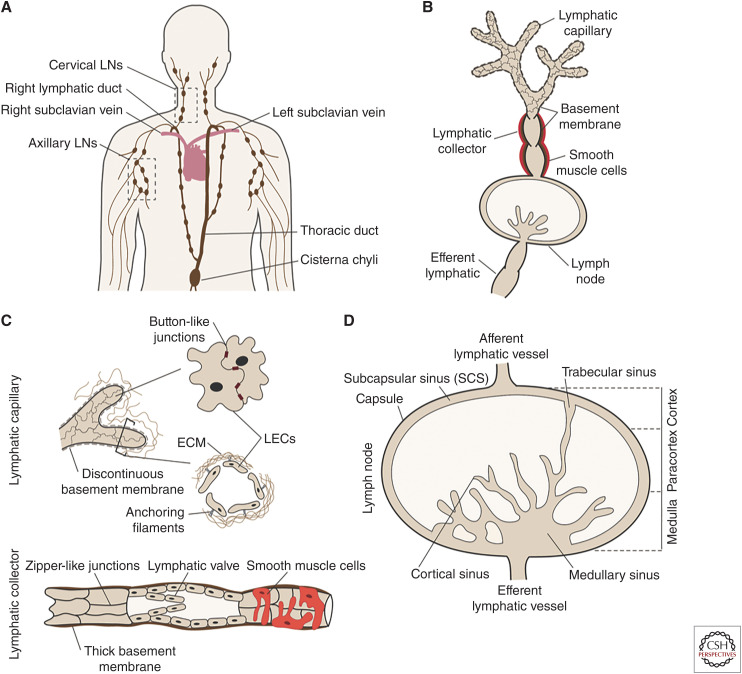
Structure of the lymphatic vasculature. (A) Lymphatic vessels (LVs) originate in peripheral tissues and are organized in a tree-like network that includes the lymph nodes (LNs). LVs eventually converge into the thoracic duct and the right lymphatic duct, which join the blood circulation at the level of the subclavian veins. (B) Afferent lymphatic capillaries in the peripheral tissues drain the lymph-containing macromolecules and cells. Lymph is then actively transported through afferent lymphatic collectors toward the draining LN (dLN) where it gets filtered. Lymph leaves the LN at the level of the efferent LV. (C) Lymphatic capillaries (upper drawing) are surrounded by a thin basement membrane and are composed of lymphatic endothelial cells (LECs) that are connected by discontinuous button-like cell–cell junctions. This arrangement generates open flaps that enable entry of lymph and leukocytes. Anchoring filaments connect LECs to the extracellular matrix (ECM) and regulate opening of the flaps. On the other hand, lymphatic collectors (lower drawing) are composed of tightly joined LECs connected by zipper-like cell–cell junctions and have a thick basement membrane. Intralymphatic valves and smooth muscle cells surrounding the collector ensure unidirectional propagation of the lymph. (D) Afferent lymphatic collectors deliver the lymph to the LN at the level of the subcapsular sinus (SCS), which surrounds the entire LN. The LN parenchyma is divided into the outer cortex, the more centrally located paracortex, and the medulla. Within the LN, the lymph flows through a network of trabecular, cortical, and medullary sinuses and exits via the efferent LV.
Lymphatic research started in the early 17th century with the discovery of the lacteal vessels, which are located in each intestinal villus and transport absorbed lipids from the small intestine to the mesenteric LNs (Aselli 1627). Over the course of the subsequent centuries, LVs were discovered in the skin, heart, lungs, and other organs (Petrova and Koh 2018, 2020; Wong et al. 2018). However, advancements in our molecular understanding of lymphatic biology were only made possible in the late twentieth century with the discovery of lymphatic-specific markers, such as the lymphatic-specific transcription factor Prox-1 (Wigle and Oliver 1999), the lymphatic vessel endothelial hyaluronan receptor 1 (LYVE-1) (Banerji et al. 1999), the mucin type-1 protein podoplanin (Breiteneder-Geleff et al. 1999), and the vascular endothelial growth factor receptor 3 (VEGFR-3) (Kaipainen et al. 1995). Their combined use is now frequently used in the identification of LVs in tissues.
ORGANIZATION OF THE LYMPHATIC VASCULATURE
The lymphatic vasculature in the body can be divided into three vascular compartments: the afferent vessels upstream of LNs, the lymphatic vasculature within LNs, and the efferent lymphatics downstream of LNs (Fig. 1B). Each of these compartments are introduced in the following paragraphs.
In peripheral tissues, a network of blind-ended afferent lymphatic capillaries make up lymph, which mainly consists of tissue fluids, leukocytes, and macromolecules. Lymphatic capillaries are composed of a single layer of partly overlapping oak leaf-shaped lymphatic endothelial cells (LECs) (Baluk et al. 2007). These cells are loosely interconnected through discontinuous button-like cell–cell junctions, giving rise to openings, so-called flaps, or primary valves (Fig. 1C), which facilitate the uptake of interstitial fluid and leukocytes into the vessel. Capillary LECs are surrounded by a thin and fenestrated basement membrane and are connected by anchoring filaments to the extracellular matrix (ECM). This setup leads to the spontaneous opening of the flaps once interstitial tissue fluid pressure increases (e.g., during inflammation) (Leak and Burke 1966).
Lymphatic capillaries converge into precollecting vessels that merge into larger collecting vessels (Moore and Bertram 2018). While capillaries and collectors start as rather small vessel segments within tissues (diameter in skin of ∼50 µm) (Suami and Scaglioni 2018; Montoya-Zegarra et al. 2019), they steadily increase in size as collecting vessels merge in the lymphatic network. The largest lymphatic collector, the thoracic duct, has been reported to have a diameter of up to 5 mm in humans (Skandalakis et al. 2007). Considering that LNs are frequently aligned in chains connected by collectors (Fig. 1A), an efferent vessel exiting from one LN can at the same time be the afferent vessel for the next dLN (Förster et al. 2012). In this article, we exclusively use the term “afferent LVs” when referring to LVs that begin in peripheral tissues. The main function of collectors lies in the transport of lymph. Regardless of their size, the transport function of collecting vessels requires distinct morphological features compared to capillaries, which primarily serve in lymph uptake. Collector LECs are connected by continuous and tight zipper-like cell–cell junctions and display an elongated shape (Fig. 1C; Baluk et al. 2007). Furthermore, collectors are surrounded by a thick continuous basement membrane (Pflicke and Sixt 2009), which together with the zipper-like junctional setup ensures fluid tightness. Lymphatic collectors are further surrounded by a smooth muscle cell layer, which confers contractility and lymph propulsion (Oliver et al. 2020; Petrova and Koh 2020). Additionally, intraluminal valves divide collecting vessels into distinct units, so-called lymphangions, which prevent backflow of lymph and ensure unidirectional flow toward dLNs (Bazigou and Makinen 2013; Moore and Bertram 2018).
Humans have up to 600 LNs distributed throughout the whole body, while mice only have around 20 (Van den Broeck et al. 2006). The complex structure of LNs is key for initiating and regulating immune responses. LNs are compartmentalized into an outer cortex, followed by a paracortex and the medulla on the inside (Fig. 1D). These compartments from the LN parenchyma are lined on all sides by a series of lymphatic sinuses through which the lymph travels. First, the lymph is delivered via one or more afferent LVs to the subcapsular sinus (SCS), a compartment continuous with the afferent lymphatics and situated just underneath the outer collagen-rich capsule of the node. Within the LN, the afferent vessels branch into a sinus system comprising multiple conduits for the intranodal trafficking of antigens and cells. Subsequently, the lymph flows through trabecular sinuses and cortical sinuses, which expand to become wider medullary sinuses (Jalkanen and Salmi 2020). All LN sinuses are lined by LECs that can be divided into different subtypes depending on their localization and function (Jalkanen and Salmi 2020). For example, the SCS is lined by specialized ceiling and floor LECs. Macrophages that are interspersed with LECs in the SCS and medullary sinuses floor sample the lymph and capture free antigens or even bacteria or viruses (Louie and Liao 2019). Alternatively, small soluble antigens (<70 kDa) can be passively transported from the SCS to the LN parenchyma via specialized reticular conduits (Chang and Turley 2015). These small, collagen-rich channels are tightly surrounded by fibroblastic reticular cells. LN-resident dendritic cells (DCs) can access antigen in these conduits and present them to lymphocytes in the LN parenchyma (Chang and Turley 2015). Larger molecules and lymph-borne antibodies that are physically unable to enter via this route may get transported by vesicular transcytosis across the floor LECs into the LN parenchyma (Kähäri et al. 2019). After traveling along the complex sinus architecture, the filtered lymph leaves the node through an efferent LV in the hilus region (Fig. 1D). Ultimately, larger collecting vessels converge into the thoracic and lymphatic ducts, which return the lymph into the blood circulation at the level of the subclavian veins.
CELLULAR MIGRATION THROUGH AFFERENT AND EFFERENT LYMPHATIC VESSELS
Seminal cannulation studies performed in the late 1950s by Sir James Gowans revealed that lymphocytes constantly recirculate between SLOs and blood (Gowans 1957). The most prominent recirculating lymphocytes are naive T and B cells, which enter and exit SLOs in search of cognate antigen, thereby contributing to immune surveillance (von Andrian and Mackay 2000; Krummel et al. 2016). Lymphocyte extravasation from the bloodstream into LNs takes place in a specialized vascular bed, the so-called high endothelial venules (HEVs). The process occurs through a multistep adhesion cascade, culminating in lymphocyte transmigration into the paracortex (Girard et al. 2012). Within the LN, T and B cells migrate toward the T-cell zone and B-cell follicles, located in the paracortex and cortex, respectively, to scan for antigens. Naive B cells directly take up antigen via their B-cell receptor, whereas naive T cells need DCs, which present antigen to them on MHC molecules. Upon antigen recognition, both lymphocytes are retained in the LN and differentiate into effector T cells or antibody-secreting plasma cells. Once differentiated, these cells leave the LN via the efferent LVs to eventually enter the bloodstream and migrate (e.g., to the site of infection) (von Andrian and Mackay 2000; Krummel et al. 2016). Conversely, if no antigen recognition occurs, the recirculation of naive T cells continues, and they exit the LN after a few hours via efferent LVs.
Since the late 1950s, cannulation techniques have commonly been used to investigate the cellular composition of the lymph in big species, such as sheep, cows, or humans (Smith et al. 1970; Bujdoso et al. 1989; Olszewski et al. 1995). These studies have revealed that lymph collected from efferent lymphatics (e.g., thoracic duct or other postnodal collection sites) contains almost exclusively lymphocytes, namely, recirculating naive T and B cells but also certain types of antigen-experienced lymphocytes (Mackay et al. 1988). Interestingly, the composition and cellular load of efferent lymph is linked to the inflammatory state of the tissue from which the lymph is drained. Upon antigenic stimulation and the concomitant inflammatory response, lymphocytes are retained in the dLN. This phase is described as “LN shutdown” and leads to lower numbers of lymphocytes in efferent lymph as well as swelling of the LNs. After a few days, increased numbers of lymphocytes (many of them differentiated effector cells) egress from the node, resulting in an elevated cellular output before the numbers decrease again back to steady-state baseline levels. This shutdown process is hypothesized to promote the likelihood of antigen-specific activation of lymphocytes in dLNs (Cahill et al. 1976; Mackay et al. 1992; Wee et al. 2011).
While efferent lymph contains a large portion of naive T and B cells, afferent lymph mostly contains antigen-experienced effector or memory T cells (80%–90%), DCs (5%–15%), as well as some granulocytes and monocytes (1%–10%), depending on the inflammatory state (Smith et al. 1970; Sokolowski et al. 1978; Zawieja et al. 2019). The best characterized cell type in afferent lymph undoubtedly are DCs, which migrate to dLNs to present antigen taken up in peripheral tissues to CD8+ or CD4+ T cells. In the context of inflammation and/or infection, migratory DCs mature and up-regulate costimulatory molecules on their cell surface, leading to efficient activation of cognate T cells in dLNs. Conversely, DCs migrating in steady-state present mostly self-antigens and express low levels of costimulatory molecules. Cognate T cells activated under these conditions typically undergo clonal deletion or are rendered anergic or tolerant, highlighting the important role of steady-state DC migration in the maintenance of peripheral tolerance. DCs that have migrated to dLNs typically are short-lived and do not traffic further but undergo apoptosis (Hilligan and Ronchese 2020).
Antigen-experienced CD4+ T cells and to a lesser extent CD8+ T cells constitute the largest leukocyte subsets in afferent lymph (Mackay et al. 1990). In steady-state conditions, the most abundant CD4+ T cells in afferent lymph are long-lived effector memory T cells (TEM) (Yawalkar et al. 2000). Functionally, TEM recirculation through peripheral tissues is thought to contribute to immune surveillance. However, in recent years, tissue-resident memory cell populations have also been detected, which fulfill this task (Mueller and Mackay 2016; Ho and Kupper 2019). Under inflammatory conditions, short-lived effector CD4+ T cells are also found in large amounts in afferent lymph. While antigen-specific T cells are thought to be retained in the inflamed/infected tissue, tissue-exit of unspecific T cells is thought to help prevent overshooting inflammatory responses in the tissue, which may otherwise be induced by bystander activation of these unspecific T cells (Gómez et al. 2015; McNamee et al. 2015). Furthermore, recent studies in murine models of inflammation have identified regulatory T cells (Tregs) as a major T-cell subset exiting tissues both in steady-state and inflammation. Tregs migration from peripheral tissues to dLNs is thought to help maintain immune tolerance and suppress and regulate the ongoing immune response (Tomura et al. 2010; Nakanishi et al. 2017).
Finally, it is important to note that the total cellular load in afferent lymph is approximately one magnitude lower than the one of efferent lymph, in line with the fact that ∼90% of all cells enter LNs via HEVs and that efferent lymph mainly comprises recirculating (naive) lymphocytes (Smith et al. 1970; Mackay et al. 1988, 1990).
METHODS USED TO STUDY LEUKOCYTE MIGRATION THROUGH THE LYMPHATIC VASCULATURE
Since the pioneering cannulation studies describing the composition of lymph, novel techniques have been developed and applied to study lymphatic migration. Particularly the development of knockout (KO) mice made it possible to investigate the involvement of a gene of interest in leukocyte migration (Fig. 2), for example, when used in combination with so-called fluorescein isothiocyanate (FITC) painting or adoptive transfer experiments (Table 1). In FITC painting, the fluorophore FITC is applied onto murine skin. After penetrating the epidermis and dermis, FITC is taken up by DCs and other cell types. FITC+ migratory DCs can subsequently be detected and quantified in dLNs (e.g., by flow cytometry performed on LN single-cell suspensions). In contrast, in adoptive transfer experiments, fluorescently labeled wild-type (WT) and/or KO leukocytes are injected into the skin or the footpad of a recipient mouse and their migration to dLNs is subsequently analyzed by immunofluorescence or flow cytometry (see Table 1). Mouse strains in which the Cre recombinase is expressed under the control of a lymphatic marker like Prox-1 or VEGFR-3 (e.g., Bazigou et al. 2009; Martinez-Corral et al. 2016) can be used to generate lymphatic-specific KO mice. Considering that many molecules involved in lymphatic trafficking are not exclusively expressed by the lymphatic vasculature, the latter mice are important for assessing the LEC-specific contribution to migration.
Figure 2.
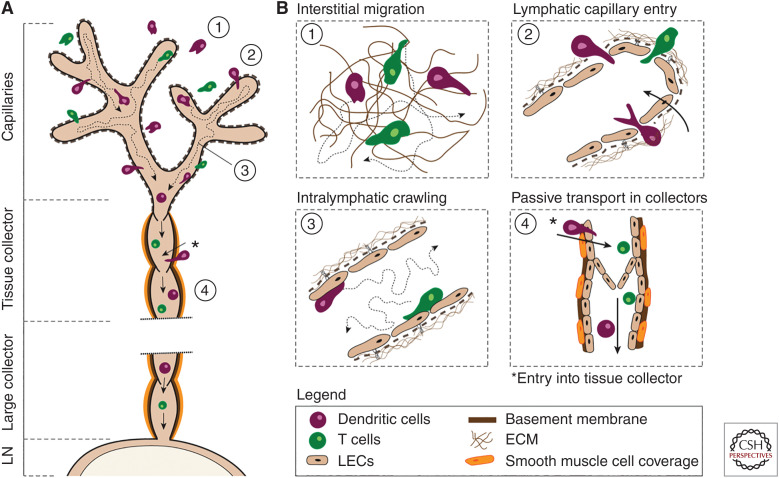
Stepwise leukocyte migration through afferent lymphatics. (A) Schematic depiction of leukocyte migration from peripheral tissues through afferent lymphatics. (B) Illustration of the stepwise leukocyte migration pattern. (1) Interstitial migration: dendritic cells (DCs) and T cells migrate within the interstitial space composed of extracellular matrix (ECM) toward initial lymphatic capillaries, guided by chemotactic cues such as CCL21. (2) Lymphatic capillary entry: DCs and T cells enter blind-ended capillaries through specialized flaps formed by capillary lymphatic endothelial cells (LECs). (3) Intralymphatic crawling: DCs and T cells actively crawl and patrol within the capillary lumen attached to the LEC surface. (4) Passive transport through collectors: Lymph flow increases in collectors due to contraction of the vessel-surrounding lymphatic smooth muscle cells (SMCs). Once leukocytes arrive in lymphatic collectors, they start to detach from the lymphatic endothelium and are passively transported toward the next lymph node (LN) by flow. *During inflammation, DCs can enter directly into lymphatic tissue collectors that provide a rapid route to draining LNs (dLNs) by bypassing the slow migration within the capillaries.
Table 1.
Methods for studying leukocyte migration
| Method | Description, advantages (+) and disadvantages (−) |
|---|---|
|
Cannulation studies Used to study: Lymph node (LN) exit (efferent lymph) Entry into afferent lymphatic vessels (LVs) (afferent lymph) |
Lymph fluid is collected via a cannula placed directly inside an afferent or efferent LV, or into the cisterna chyli. Its cellular content is subsequently characterized by microscopy or flow cytometry. Cannulation studies are typically performed in larger animals/humans, and more rarely also in rodents (Gowans 1957; Mackay et al. 1988; Yawalkar et al. 2000; Zawieja et al. 2019).
|
|
Adoptive transfer (I) (e.g., injected subcutaneously or into the footpad) Used to study: Entry into afferent LVs Entry from afferent LVs into LNs |
Fluorescently labeled or otherwise marked leukocytes (e.g., expressing a fluorescent protein or congenic marker) are injected into peripheral tissues of recipient mice and their presence in the draining LN (dLN) is subsequently quantified by flow cytometry or immunofluorescence. Common sites of injection are the ear skin or footpad, as they mainly drain into one single LN (i.e., the auricular or popliteal LN, respectively). In some cases, intralymphatic injection is also used (Ohl et al. 2004; Johnson et al. 2006; Brinkman et al. 2016; Teijeira et al. 2017; Martens et al. 2020).
To study the residence time of T cells in LNs, a special adoptive transfer setup is used. Specifically, fluorescently labeled T cells are first transferred intravenously into a recipient mouse from where they home to LNs. After a few hours, further immigration of T cells into LNs via high endothelial venules (HEVs) is blocked by administration of entry-blocking antibodies (e.g., anti-integrin α4 or anti-L-selectin). In this way, T-cell entry is uncoupled from egress, which will continue to occur. To quantify LN egress, the number of fluorescent T cells in the LNs at the time of antibody injection is determined by quantitative flow cytometry and compared to the number found in the LNs in the control and the treatment group (or wild-type [WT] vs. knockout [KO]) at a certain end point (e.g., 24 h). If egress is inhibited, more T cells should be present in the LN in the treated (or KO) group at the end point (Halin et al. 2005; Pham et al. 2010; Reichardt et al. 2013).
|
|
Adoptive transfer (II) Intravenous injection Used to study LN egress | |
|
FITC/TRITC painting Used to study: Entry into afferent LVs Entry from afferent LVs into LNs |
Topical or intranasal/intratracheal administration of fluorescein isothiocyanate (FITC) or TRITC allows analysis of the endogenous migration of DCs from the skin or lung, respectively. FITC or TRITC is taken up by dermal or pulmonary DCs, which subsequently can be identified in dLNs by flow cytometry or microscopy (Förster et al. 1999; Qu et al. 2004; Vigl et al. 2011; Iolyeva et al. 2013).
|
|
Photoconvertible transgenic mice Used to study: Migration via afferent LVs to LNs |
Performed in mice ubiquitously expressing a photoconvertible green- fluorescent protein (e.g., Kikume or Kaede protein), which is converted to a red-fluorescent state upon illumination with (ultra)violet light. Migratory photoconverted cells can be tracked by their red fluorescence and detected and quantified by flow cytometry in tissues like LNs (Tomura et al. 2008, 2010; Bromley et al. 2013; Tadayon et al. 2021).
|
|
Intravital microscopy Used to study: Entry/migration within afferent LVs Entry into or exit from LNs across lymphatic sinuses |
Fluorescent reporter mice adoptively transferred fluorescently labeled leukocytes or injected fluorescent antibodies are used to visualize LVs and leukocytes in vivo. The migration of leukocytes is studied at the single-cell level by time-lapse confocal or multiphoton microscopy to determine (e.g., the speed, directionality, or cellular interactions of migrating cells). Common imaging sites are the popliteal LNs, ear skin, or footpads (Grigorova et al. 2010; Tal et al. 2011; Teijeira et al. 2017; Hunter et al. 2019).
|
|
Skin explants Used to study: Migration toward, into, and within afferent LVs |
Murine ear skin is ripped along the central cartilage and fluorescently labeled leukocytes are added on top of the luminal side of the explant and left to migrate into the dermal tissue. Migration into afferent lymphatics is analyzed after ∼1–4 h by confocal microscopy after staining LVs with fluorescent antibodies. Alternatively, migration of fluorescent leukocytes can be assessed in real time by performing time-lapse imaging in explants with fluorescent LVs (either stained by antibodies or endogenously visualized in LV reporter mice) (Lämmermann et al. 2008; Teijeira et al. 2013; Russo et al. 2016; Johnson et al. 2017).
|
|
In vitro assays Used to study: Adhesion Transmigration Crawling |
Specific migration steps, such as transmigration, adhesion, or crawling, can be investigated by in vitro functional assays using lymphatic endothelial cell (LEC) monolayers and ex vivo isolated or in vitro differentiated/activated leukocytes (Ledgerwood et al. 2008; Russo et al. 2016; Arasa et al. 2021; Johnson et al. 2021).
|
In addition to adoptive transfer and FITC painting experiments, photoconversion experiments are increasingly used for investigating molecules involved in leukocyte migration through afferent LVs. The latter rely on transgenic mice expressing a green fluorescent, photoconvertible protein, such as Kikume green-red or Kaede, in all cell types (Tomura et al. 2008, 2014). These proteins undergo a conversion to the red-fluorescent state upon illumination with ultraviolet or violet light. By inducing photoconversion in a particular tissue (e.g., area of the skin), the red-fluorescent cells originating from this illuminated tissue can be distinguished from all other (green-fluorescent) cells in the animal. The technique therefore allows researchers to identify and quantify red-fluorescent leukocytes that have migrated from the illuminated tissue via afferent lymphatics to dLNs and to investigate the mechanism of their migration in an endogenous setup (see, e.g., Tomura et al. 2008; Bromley et al. 2013; Hampton et al. 2015; Ikebuchi et al. 2016; Tadayon et al. 2021).
Another important advancement in our understanding of leukocyte migration through LVs has come from intravital microscopy (IVM), which involves imaging leukocyte migration in vivo in anesthetized mice by time-lapse confocal or multiphoton microscopy. These imaging techniques allow researchers to investigate the migratory patterns and interactions between different cells inside LVs, dLNs, or tissue (e.g., skin) with single-cell resolution (Secklehner et al. 2017; Hunter et al. 2019; Nakamizo et al. 2020). In contrast to, for example, adoptive transfer or photoconversion experiments, which investigate the mechanisms of lymphatic migration at the population level, analysis at the single-cell level allows researchers to identify migration cascades and to study the involvement of a gene of interest in a particular migratory step. IVM generally requires the use of reporter mice that feature cell-type-specific expression of a fluorescent protein in LECs (Choi et al. 2011; Hägerling et al. 2011; Bianchi et al. 2015) and DCs (Lindquist et al. 2004) or T cells (Veiga-Fernandes et al. 2007).
Besides in vivo models, also in vitro functional assays are useful for exploring different aspects of migration, such as leukocyte chemotaxis, adhesion, transmigration, or crawling on LECs (see, e.g., Johnson et al. 2006; Russo et al. 2016; Arasa et al. 2021). Nowadays, LECs can be bought commercially or isolated from different human and murine tissues, which provides a particular advantage when working with WT and KO mice (Hirosue et al. 2014; Frye et al. 2018). Alternatively, genetic knockdown can also be achieved by viral transduction or transfection of LECs and combined with in vitro migration experiments (McKimmie et al. 2013; Tadayon et al. 2021).
Migration experiments performed in murine tissue explants represent an attractive intermediate between in vitro and in vivo experiments, as they allow the study of different migration steps in a 3D tissue environment. A frequently used method is the ex vivo crawl-in assay originally described by Lämmermann et al. (2008). In this assay, fluorescently labeled DCs or T cells in medium are added onto murine ear skin halves, where they within a short time (<1 h) start to migrate into the tissue and toward/into LVs. Using time-lapse microscopy, this setup—similarly to IVM—allows one to investigate this process at the single-cell level (e.g., to determine the chemotactic requirements of migration), the process of cellular transmigration, or leukocyte movement within LVs (Pflicke and Sixt 2009; Weber et al. 2013; Russo et al. 2016; Arasa et al. 2021). On the other hand, by taking end point images 1–4 h after addition of the leukocytes (i.e., at time points where many of them have already entered into the lymphatic vasculature), the assay allows one to quantitatively assess the contribution of a particular LEC- or leukocyte-expressed molecule in migration into LVs (Weber et al. 2013; Arasa et al. 2021).
A more detailed description of the aforementioned techniques can be found in Table 1.
STEPWISE MIGRATION OF LEUKOCYTES THROUGH AFFERENT LYMPHATIC VESSELS
In analogy to the blood vascular system, the propagation of leukocytes in afferent lymphatics was, until recently, assumed to occur exclusively in a passive manner (i.e., mediated by flow). However, lymph flow in initial lymphatic capillaries is much lower (average velocities of 3–5 µm/sec) (Berk et al. 1996; Swartz et al. 1996) in comparison to the flow in, for example, blood postcapillary venules) (∼1 mm/sec) (Jiang et al. 1999). This explains why leukocytes that have entered into dermal afferent lymphatic capillaries continue to actively migrate within the vessel lumen and only start to be passively transported with flow once they reach vessel segments of higher flow, namely, the contracting collecting vessels. In the following, this stepwise migration cascade shall be discussed in greater detail (Fig. 2). Notably, most of these findings come from IVM performed in dermal lymphatics, as these are easily imaged by IVM.
Figure 3.
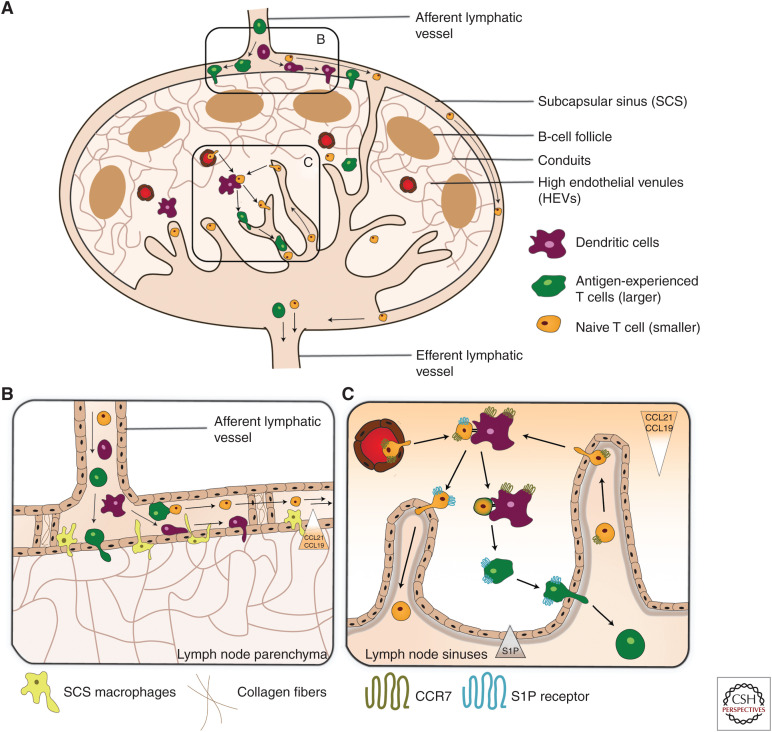
Migration into draining lymph node (dLN) and exit via efferent lymphatics. Schematic illustration of (A) Leukocyte migration into and from LNs. Leukocytes enter LNs through high endothelial venules ([HEVs]; mostly T cells and B cells) or via afferent lymphatic vessels ([LVs]; mostly antigen-experienced T cells and dendritic cells [DCs]). Once in the LN parenchyma, DCs and T cells migrate into the T-cell zone and B cells into the B-cell zone, guided by chemokines. Cellular exit from LNs exclusively occurs via the lymphatic sinuses and the efferent LV. (B) Entry of lymph-borne leukocytes across the subcapsular sinus (SCS). The SCS is formed by a ceiling and floor composed of lymphatic endothelial cells (LECs) and, in the case of the floor, also SCS macrophages. The SCS lumen is interspersed with pillar-like trans-sinusoidal tissue cords composed of collagen fibers and surrounding LECs. This creates a sieve-like meshwork that only allows the free passage of smaller cells, such as naive T cells. These are passively propagated toward the medullary sinuses. Larger cells, such as DCs or activated T cells, are retained in the SCS meshwork and actively transmigrate into the LN across the SCS. Their transmigration is guided by their CCR7 receptor that senses the CCL21/CCL19 gradient directed into the T-cell zone. (C) Leukocyte entry and exit across the medullary sinuses. Small (naive) T cells arriving via the afferent LVs enter the LN by crossing the medullary sinuses. While transmigration occurs independently of CCR7, intranodal migration toward the T-cell zone is highly CCR7-dependent. Leukocyte exit is guided by the steep S1P gradient formed between the LN parenchyma and lymph. S1PR1-expressing T cells sense this gradient when in proximity of the S1P-producing LECs lining the cortical and medullary sinuses. This induces their transmigration into the lymphatic sinus system and egress from the LN via the efferent LV.
Most leukocytes are not immotile within tissues but display low levels of interstitial migration. While leukocytes within uninflamed tissue often migrate in a random undirected manner, leukocytes approaching sites of tissue damage or infections typically follow chemotactic cues (Kienle and Lämmermann 2016). Similarly, leukocytes follow chemokine gradients when approaching afferent lymphatics (Lämmermann et al. 2008; Weber et al. 2013). To enter into the lumen of lymphatic capillaries, leukocytes preferentially transmigrate through the open flaps formed by neighboring LECs (Pflicke and Sixt 2009). Upon entering the afferent LVs, leukocytes stay attached on the luminal side of the capillaries where they actively crawl and migrate on the surface of LECs (Tal et al. 2011; Teijeira et al. 2017). During steady-state conditions, leukocytes within capillaries crawl at a speed of around 5–10 µm/sec (Russo et al. 2016; Teijeira et al. 2017). Interestingly, leukocytes spend many hours patrolling within capillaries or even remain arrested for longer time periods (Hunter et al. 2019). Arriving in contracting tissue collectors, they can be observed rolling along the vessel wall at low speed (1 mm/min) (Teijeira et al. 2017), but once they reach larger collectors, leukocytes start to detach and can flow at speeds of up to several mm/sec, as observed in the mesentery (Dixon et al. 2006; Akl et al. 2011). This transition from active crawling to passive flowing is likely due to increased lymph flow rates and is extremely important to ensure timely arrival of cells in dLNs.
Intriguingly, leukocyte entry into afferent lymphatics is not solely limited to capillaries. Our laboratory has recently found that under inflammatory conditions DCs may directly enter tissue collectors, thereby bypassing the capillary compartment (Arasa et al. 2021). Specifically, in inflammation, the thick basement membrane and collagen layer surrounding lymphatic collectors are degraded, and certain adhesion molecules important for DC trafficking preferentially up-regulated in collector LECs, supporting DC access and entry into tissue collectors. This alternative route of migration bypasses the slow, active crawling step in capillaries, thereby enabling DCs to arrive more rapidly in dLNs (Arasa et al. 2021). The purpose of this alternative route of migration remains to be determined.
MOLECULES INVOLVED IN DC AND T-CELL MIGRATION THROUGH AFFERENT LYMPHATICS
In the following section we describe the most important molecules involved in DC and T-cell migration through afferent lymphatics. Although several of the mentioned molecules reportedly also mediate migration of other cell types, in particular of neutrophils (Jackson 2019; Schineis et al. 2019), we here solely focus on DCs and T cells, since they are the most abundant and best-studied cell types migrating through afferent LVs.
Chemotactic Guidance
CCL21-CCR7 Signaling Axis
Probably the most important molecules involved in DC and T-cell migration to dLNs under both steady-state and inflammatory conditions are DC/T-cell-expressed C-C Motif Chemokine Receptor 7 (CCR7) and its LEC-expressed C-C Motif Chemokine Ligand 21 (CCL21) (for review, see Förster et al. 2008). CCR7 is expressed—at least at low levels—by most peripheral T-cell subsets (Clark et al. 2006; Bromley et al. 2013; Watanabe et al. 2015) and is up-regulated on DCs upon activation (Sallusto et al. 1998; Ohl et al. 2004). CCL21 is constitutively expressed by LECs of afferent lymphatics (Johnson and Jackson 2010; Weber et al. 2013), with greater expression in capillaries than in collectors (Russo et al. 2016; Arasa et al. 2021). Because of its positively charged carboxy terminus, CCL21 binds to negatively charged structures, such as heparan sulfate (HS) proteoglycans present on LECs, and in the ECM (Bao et al. 2010). Consequently, CCL21 forms a steep perilymphatic gradient, which guides DCs from distances of up to 90 µm toward lymphatic capillaries (Weber et al. 2013). Curiously, LEC-produced HS is dispensable for the formation of the interstitial CCL21 gradient and DC migration toward capillaries (Vaahtomeri et al. 2021), suggesting CCL21's major anchoring site to be the ECM surrounding lymphatic capillaries or other molecules expressed on LECs (e.g., possibly podoplanin) (Kerjaschki et al. 2004). In addition to the immobilized full-length CCL21, a soluble version lacking the carboxy terminus (CCL21-ΔC), which can be cleaved by plasmin, exists (Lorenz et al. 2016). Consequently, leukocytes migrate along immobilized chemokine gradients in a migratory process called haptotaxis and/or along soluble chemokine gradients (chemotaxis). The availability of both CCL21 forms was shown to be regulated by the scavenging receptor ACKR4 (atypical chemokine receptor 4) expressed on stromal cells, particularly keratinocytes and fibroblasts (Bryce et al. 2016; Bastow et al. 2021). In ACKR4-deficient mice, CCL21 accumulates in the tissue, leading to reduced DC entry into capillaries (Bastow et al. 2021). Interestingly, genetic deletion of C-C motif chemokine ligand 19 (CCL19), the second ligand of CCR7 (Förster et al. 2008), completely rescued lymphatic trafficking of DCs in ACKR4-deficient mice during inflammation (Bryce et al. 2016). Notably, CCL19 is not expressed by LECs but rather by mature DCs, as well as fibroblastic reticular cells (Förster et al. 2008). These findings suggest that ACKR4 might be facilitating CCR7-dependent DC trafficking by scavenging DC-produced CCL19, which would otherwise confound the sensing of CCL21 gradients in the tissue (Bryce et al. 2016). The spatiotemporal availability of CCL21 is also impacted by other factors such as inflammatory cytokines (Johnson and Jackson 2010) and DC interactions with LECs (Schumann et al. 2010). In addition to guiding leukocyte migration from the interstitium toward the afferent lymphatics, our group found that CCL21 also promoted the semidirected migration of DCs once within lymphatic capillaries (Russo et al. 2016). Our analyses suggested that the low lymph flow within capillaries establishes a downstream-oriented CCL21 gradient along the capillary LECs. DCs actively crawling within the capillaries frequently change direction, but overall preferentially follow the flow-induced CCL21 gradient in the direction of the downstream collecting vessels (Russo et al. 2016). Although better studied for DCs, lymphatic migration of T cells also highly depends on the expression of CCR7 (Debes et al. 2005; Bromley et al. 2013).
S1P Signaling Axis
Another chemotactic pathway contributing to DC and T-cell migration into initial lymphatics involves the chemotactic sphingolipid sphingosine-1-phosphate (S1P). S1P levels are high in blood and lymph but low in tissues including LNs (Baeyens and Schwab 2020). In the lymphatic vasculature, S1P is produced by LECs (Pham et al. 2010) and can signal via five known G-protein-coupled S1P receptors (sphingosine-1-phosphate receptors 1-5 [S1PR1-5]), which are differentially expressed on leukocytes and (e.g., endothelial cells and induce a variety of downstream signaling events). T cells and maturing DCs express different types of S1PR1-5 (Baeyens and Schwab 2020); however, most research has focused on S1PR1 signaling in T cells. Interaction of S1P with T-cell-expressed S1PR1 or S1PR4 promotes migration of T cells from peripheral tissues into initial lymphatics (Brown et al. 2010; Xiong et al. 2019). Inhibition of S1PR1 signaling, using the functional antagonist FTY720, resulted in accumulation of T cells in peripheral tissues near LVs and reduced T-cell migration to dLNs (Brown et al. 2010; Xiong et al. 2019). Notably, S1P not only acts on T cells but also on LECs: S1P signaling via LEC-expressed S1PR2 was shown to regulate the expression of the adhesion molecule VCAM-1, thereby impacting T-cell trafficking (Xiong et al. 2019). In this context, it is also worthwhile mentioning the T-cell-expressed surface protein CD69, which is known to destabilize and induce degradation of S1PR1. Consequently, CD69 expression blocks S1P-mediated T-cell egress from peripheral tissues. In fact, most tissue-resident memory T cells express CD69, as it results in their retention, allowing these cells to mediate local immune protection in, for example, the skin and mucosa (Mackay et al. 2015). Even though the S1P pathway is less well studied in DCs than in T cells, signaling via S1PR1 was shown to also contribute to DC migration from skin and lung to dLNs (Czeloth et al. 2005; Gollmann et al. 2008), whereas S1PR3 has been implicated in DC migration from the intestine to mesenteric LNs (Karuppuchamy et al. 2017).
Besides CCR7 and S1P signaling, several other chemotactic cues influencing leukocyte migration in afferent lymphatics have been described and are summarized in Table 2.
Table 2.
Molecules involved in regulating leukocyte entry and trafficking through afferent lymphatics toward draining lymph nodes (dLNs)
| Molecules |
||
|---|---|---|
| Expressed by LECs | Expressed by interacting cell | Comments |
| CCL21/CCL19 | CCR7 Dendritic cells (DCs), T cells |
CCL21 is expressed by LECs and forms interstitial gradients that guide DCs and T cells expressing CCR7 toward the afferent lymphatics (Bromley et al. 2005; Debes et al. 2005; Weber et al. 2013). CCL21 also forms intralymphatic gradients that guide CCR7+ DCs within the afferent capillaries in the downstream direction of the dLNs (Russo et al. 2016). |
| ACKR4 Keratinocytes Fibroblasts Lymphatic endothelial cells (LECs) |
CCR7 DCs |
The scavenger receptor ACKR4 expressed by dermal keratinocytes, fibroblasts, and a subset of LECs facilitates CCR7-dependent DC trafficking from skin into lymphatic capillaries and LNs by scavenging CCL19 (Bryce et al. 2016) and impacting the formation of CCL21 gradients (Bastow et al. 2021). |
| CCL21 | PD-L1 (CCR7) DCs |
PD-L1 intracellular signaling controls DC migration from skin to dLNs by regulating CCR7-mediated chemotaxis (Lucas et al. 2020). |
| S1P | S1PR1/S1PR3 DCs, T cells |
S1P is produced by LECs and present at higher levels in lymph than in tissues (Pham et al. 2010). Blocking or deficiency of S1P receptors expressed on DCs and T cells reduces their migration from skin to dLNs (Czeloth et al. 2005; Gollmann et al. 2008; Karuppuchamy et al. 2017; Xiong et al. 2019). |
| CD69 T cells |
CD69 expressed on T cells down-regulates S1PR1 and is critical for prolonged T-cell retention and local memory formation in the tissues (Mackay et al. 2015). | |
| CXCL12 | CXCR4 DCs |
The chemokine CXCL12 expressed by LECs mediates cutaneous DC migration to dLNs in steady-state and inflammation (Kabashima et al. 2007). |
| CX3CL1 | CX3CL1R DCs |
LEC-expressed chemokine CX3CL1 is up-regulated in inflamed lymphatics and promotes trafficking of DCs to LNs (Johnson and Jackson 2013). |
| CCL1 | CCR8 DCs |
C-C Motif Chemokine Ligand 1 (CCL1) is up-regulated in inflamed lymphatics supporting migration of C-C Motif Chemokine Receptor 8 (CCR8)-expressing DCs to dLNs (Qu et al. 2004; Sokol et al. 2018). |
| Semaphorin3a | Plexin-A1 and Neuropilin-1 DCs, T cells |
LEC-expressed semaphorin3a induces actomyosin contraction in DCs thus easing DC entry into afferent lymphatics and migration to dLNs (Takamatsu et al. 2010). |
| Integrin ligands | Integrin ligands such as ICAM-1 and VCAM-1 are expressed at low levels by LECs in steady-state but get up-regulated in inflammation (Johnson et al. 2006; Vigl et al. 2011). DCs and T cells express integrins LFA-1 and VLA4, which bind to ICAM-1 or VCAM-1, respectively. Their migration is integrin independent in steady-state likely due to the low expression of integrin ligands but becomes integrin dependent under inflammatory conditions (Johnson et al. 2006; Lämmermann et al. 2008; Arasa et al. 2021). | |
| ICAM-1 | LFA-1 DCs, T cells |
|
| VCAM-1 | VLA4 DCs, T cells |
|
| L1CAM | L1CAM DCs |
L1 Cell Adhesion Molecule (L1CAM) expression on DCs or LECs helps trafficking from the skin to dLNs (Maddaluno et al. 2009). |
| ALCAM | ALCAM DCs, T cells |
Activated Leukocyte Cell Adhesion Molecule (ALCAM) supports DC transmigration and crawling on LECs. DC migration to lung-dLNs is reduced in ALCAMKO mice (Iolyeva et al. 2013; Willrodt et al. 2019). |
| CLEVER-1 | Unknown ligand DCs and T cells |
CLEVER-1 supports DC and T-cell migration to dLNs (Salmi et al. 2004; Karikoski et al. 2009; Tadayon et al. 2021). |
| LYVE-1 | CD44 DCs |
LYVE-1 expressed by capillary LECs supports docking of DCs to LECs and migration to dLNs through hyaluronan-mediated interactions (Johnson et al. 2017). CD44 controls the density of the hyaluronan glycocalyx, thus regulating the efficiency of DC trafficking to LNs (Johnson et al. 2021). |
| Macrophage mannose receptor (MMR) | CD44 T cells |
Interaction of MMR on LECs with CD44 on T cells supports T-cell migration via afferent LVs (Marttila-Ichihara et al. 2008; Salmi et al. 2013). |
| Podoplanin | CLEC-2 DCs |
DCs deficient for CLEC-2 show reduced crawling on podoplanin-expressing vessels and impaired migration to dLNs (Acton et al. 2012). |
| LTβR | LTαβ Treg cells |
Tregs use lymphotoxin αβ (LTαβ) to stimulate lymphotoxin β receptor (LTβR) on LECs, thereby inducing VCAM-1 and inflammatory chemokine expression for migration from afferent lymphatics to dLNs (Brinkman et al. 2016; Piao et al. 2018). |
| Rho-associated protein kinase (ROCK) DCs | ROCK mediates DC nuclear contraction and de-adhesion from LEC-expressed integrin ligands, thereby supporting intralymphatic DC crawling and migration to dLNs (Nitschké et al. 2012). | |
Adhesion Molecules
Apart from chemotactic cues, several adhesion molecules reportedly regulate entry of DCs and T cells into afferent lymphatics. Integrins are important for leukocyte extravasation from blood vessels (e.g., from HEVs into LNs), since they mediate firm adhesion in the vasculature and endothelial transmigration (Nourshargh and Alon 2014). Somewhat surprisingly, DC and T-cell migration through LVs to dLNs does not require integrins in steady-state (Lämmermann et al. 2008), but becomes integrin-dependent under inflammatory conditions (Johnson et al. 2006; Vigl et al. 2011; Arasa et al. 2021). The explanation for this likely is that in steady-state, LVs—in contrast to blood vessels—express virtually no integrin ligands. In inflammation, LECs undergo striking transcriptional changes and up-regulate integrin-ligands like ICAM-1 and VCAM-1 (i.e., the ligands of DC- and T-cell-expressed integrins LFA-1 and VLA-4 (Johnson et al. 2006; Vigl et al. 2011; Arasa et al. 2021). In the case of VCAM-1, we found this adhesion molecule to be preferentially up-regulated in dermal lymphatic collectors, where it mediated entry of DCs directly into collecting vessel segments (Arasa et al. 2021). Additionally, the LEC-expressed common lymphatic endothelial and vascular endothelial receptor 1 (CLEVER-1) was shown to mediate both DC and T-cell migration through afferent LVs (Karikoski et al. 2009; Tadayon et al. 2021).
Several other molecules that mediate either DC or T-cell trafficking through afferent LVs have been described, such as the lymphatic-specific hyaluronan receptor LYVE-1, a key component of endothelial junctions. LYVE-1 docks DCs to the lymphatic endothelium via hyaluronan bound to DC-expressed CD44, thereby mediating DC entry into lymphatic capillaries (Johnson et al. 2017, 2021). Similarly, interaction between LEC-expressed macrophage mannose receptor (MMR) and CD44 was shown to support the egress of CD4+ and CD8+ T cells from the skin (Marttila-Ichihara et al. 2008; Salmi et al. 2013). Further adhesion molecules contributing to DC and T-cell migration can be found in Table 2.
DC AND T-CELL ENTRY INTO dLNs FROM AFFERENT LVs
LNs are located at strategic points of the mammalian body for optimal immunosurveillance and induction of adaptive immune responses. Leukocytes can enter LNs either via afferent LVs or via HEVs (Fig. 3A; Girard et al. 2012). In the following section, entry via afferent LVs is discussed in greater detail.
This process starts with the flow of afferent lymph within the SCS. In contrast to previous assumptions, the SCS is not an open space that leukocytes freely flow through but rather has a complex 3D structure (Fig. 3B). Pillar-like trans-sinusoidal tissue cords, made up of SCS ceiling or floor LECs that surround collagen fibers, create a meshwork inside the SCS space, which limits cellular passage (Jalkanen and Salmi 2020; Martens et al. 2020). Smaller cells, such as naive T cells, can pass through this sieve and consequently arrive in the LN medulla from where they enter the LN parenchyma (Braun et al. 2011). By contrast, larger cells like DCs or activated T cells are retained in the SCS meshwork and transmigrate through the SCS floor toward the paracortical T- and B-cell areas (Martens et al. 2020).
Besides the anatomic location of entry, the contribution of trafficking molecules to leukocyte transmigration into LNs across the lymphatic sinuses has also been investigated in recent years. Studies performed with DCs lacking expression of integrins or talin1, an intracellular molecule essential for all integrin-mediated migration steps, revealed no requirements for integrins in DC migration across the SCS in steady-state (Lämmermann et al. 2008). Similarly, integrin β1 was also dispensable for DC migration across the SCS during inflammation (Arasa et al. 2021).
By intralymphatic injection of fluorescent leukocytes directly into the afferent LV upstream of the murine popliteal LN (located in the pit of the knee), the team of Reinhold Förster has been able to image movement and transmigration across the SCS at the single-cell level and to study the molecular requirements of these processes (Braun et al. 2011; Martens et al. 2020; de Castro Pinho and Förster 2021). These studies have revealed that, similarly to DCs, T cells transmigrate across the SCS integrin-independently and that their retention within the SCS is not dependent on adhesion molecules but exclusively dependent on cell size (Martens et al. 2020). With regard to chemotactic requirements, CCR7−/− DCs were found to exhibit a profound impairment in transmigration across the SCS (Braun et al. 2011). In the case of T cells, CCR7 was necessary for T-cell entry from the SCS, whereas transmigration across the medullary sinuses reportedly occurs independently of CCR7 (Braun et al. 2011; Martens et al. 2020). However, regardless of the entry site, once within the LN parenchyma, all T cells and DCs heavily relied on CCR7 for their migration into the CCL19-/CCL21-rich T-cell zone.
The chemokine gradient established by the high levels of CCL19/CCL21 present in T-cell zones was shown to be further shaped by ACKR4 expressed by LECs forming the ceiling of the SCS (Ulvmar et al. 2014). In analogy to its previously discussed scavenging function in the skin, ACKR4 was shown to remove CCL19 and CCL21 from the SCS, thereby steepening the gradient between the SCS and the LN parenchyma (Ulvmar et al. 2014). In ACKR4 KO mice, DCs fail to transmigrate into the LN parenchyma and accumulate in the SCS, whereas entry of T cells is only minimally affected by the absence of ACKR4 (Ulvmar et al. 2014; Martens et al. 2020).
LYMPHOCYTE EGRESS FROM dLNs
Regardless of their entry route (i.e., afferent LVs or HEVs), all lymphocytes exit LNs via efferent LVs. This migration step largely depends on S1P, which also mediates T-cell entry into afferent lymphatics (Fig. 3). S1P is produced by blood vascular endothelial cells, erythrocytes, platelets, and LECs and is present at much higher concentrations in blood and lymph compared to lymphoid tissues. This creates a steep S1P gradient between the LN parenchyma and lymph/blood (Yanagida and Hla 2017; Baeyens and Schwab 2020). The production of S1P depends heavily on the LEC-expressed sphingosine kinase 1 and 2, and the gradient, especially in lymphoid tissues, is shaped by continuous degradation of S1P in the LN parenchyma by S1P lyase (Dixit et al. 2019; Baeyens and Schwab 2020). The high amount of S1P in lymph and in blood desensitizes T and B cells and induces down-regulation of the S1PR1 (Lo et al. 2005; Pham et al. 2010). Naive T cells in blood therefore express little S1PR1 but high levels of CCR7, which drives their extravasation across HEVs and intranodal migration toward the CCL21- and CCL19-rich T-cell zone (Baeyens and Schwab 2020).
Once in the LN, lymphocytes start to up-regulate S1PR1 as a consequence of the low S1P levels in the LN parenchyma. In absence of antigenic stimulation, continual up-regulation of S1PR1 on T cells leads to their eventual egress (Matloubian et al. 2004; Thangada et al. 2010). Unlike the CCL21/CCL19, the S1P gradient is not far reaching, therefore the guidance by S1P is only induced once T cells are in close contact with the S1P-producing LECs of the cortical and medullary sinuses. IVM studies have shown that the flow inside the cortical sinuses support the S1P-dependent egress of lymphocytes and their advancement to the medullary sinuses (Grigorova et al. 2010; Baeyens and Schwab 2020). On the other hand, if a T cell recognizes its cognate antigen in the LN, the up-regulation of S1PR1 is inhibited by the up-regulation of CD69, which mediates degradation of S1PR1. The retention typically lasts until the differentiation of T and B cells is completed (3–5 d) and lymphocytes start to regain S1PR1 expression, allowing them to leave the LN via efferent lymphatics (Fig. 3C; Baeyens and Schwab 2020).
Besides being the site of lymphocyte egress, the medullary sinuses also function as an entry site for naive T cells entering via afferent LVs. This dual role is achieved through the fine balance between S1P/S1PR1 and CCR7/CCL21/CCL19 signaling axes. Similarly, to the situation in blood, S1PR1 is down-regulated on lymphocytes entering from lymph, due to high S1P content in afferent lymph. Consequently, CCL21 guides the entering T cells toward the T-cell zone. Conversely, once T cells have spent time in the LN scanning for antigen or differentiating into effector cells, they regain S1PR1 expression that overrides the retention mediated by CCR7 and induces their egress from the LN (Pham et al. 2008; Förster et al. 2012; Baeyens and Schwab 2020).
Recent studies have shown that CCR7-mediated retention of T cells is also supported by integrin complex LFA-1 and other chemokine receptors, such as C-X-C chemokine receptor type 4 (CXCR4). In studies with LFA-1 KO T cells, an increased LN egress rate has been observed (Fig. 3C; Katakai et al. 2013; Reichardt et al. 2013). Additionally, the scavenging receptor CLEVER-1, which is expressed by the blood endothelium and LECs, was shown to regulate both lymphocyte entry via HEVs and exit into efferent lymphatics (Irjala et al. 2003). Further molecules implicated in lymphocyte egress are summarized in Table 3.
Table 3.
Molecules involved in lymph node (LN) entry and LN egress via lymphatic vessels (LVs)
| Expressed by LN lymphatic endothelial cells (LECs) | Expressed by interacting cell | Comments |
|---|---|---|
| Molecules involved in LN entry via afferent lymphatics | ||
| CCL21/CCL19 | CCR7 DCs, T cells |
CCL21 and CCL19 direct dendritic cells (DCs) and larger T cells expressing the CCR7 receptor across the subcapsular sinus (SCS). Transmigration of naive T cells across the medullary sinuses reportedly occurs CCR7-independently. All T cells and DCs rely on CCR7-mediated migration for intranodal trafficking into the T-cell zone (Braun et al. 2011; Martens et al. 2020). |
| ACKR4 LECs of SCS Ceiling |
ACKR4 expressed on LECs of the SCS ceiling scavenges CCL21 and CCL19 from the SCS space, thereby shaping a steep CCL21/CCL19 chemotactic gradient that increases toward the paracortex. DC entry into the LN from the SCS is profoundly impaired in ACKR4KO mice, whereas T-cell entry is only minimally affected (Ulvmar et al. 2014; Martens et al. 2020). | |
| Molecules involved in LN egress across lymphatic sinuses | ||
| S1P | S1PR1 Lymphocytes |
S1P is the master regulator of lymphocyte egress from LNs. The low levels of S1P present in lymphoid tissue induces the up-regulation of S1PR1 on lymphocytes in the LN. Once in proximity of S1P-producing LECs, S1PR1-expressing lymphocytes sense the high S1P level in lymph, which overrides the retention signal mediated by CCR7. S1P thus induces transmigration across the cortical and medullary sinuses and LN egress via the efferent LV (Matloubian et al. 2004; Baeyens and Schwab 2020). |
| CD69 Transiently on activated T cells |
Upon activation, T cells transiently express the early activation marker CD69. CD69 induces the down-regulation of S1PR1 and supports the retention of T cells in LN. Once differentiated, T cells lose CD69 and regain S1PR1 expression, prompting egress from the LN (Shiow et al. 2006; Baeyens and Schwab 2020). | |
| Notch T cells |
Notch expression in T cells contributes to up-regulation of S1PR1 after T-cell differentiation. Consequently, adoptively transferred NotchKO T cells display reduced LN egress (Tindemans et al. 2020). | |
| IL4RA | The LEC-expressed IL4RA was shown to support egress of activated T cells from LNs in a model of systemic sclerosis. LEC-specific deletion of IL4RA results in a lower expression of S1P kinase 1 in LECs and correspondingly lower S1P levels in lymph (Urso et al. 2016). | |
| Integrin α9 | Stimulation of LEC-expressed integrin α9 via tenascin-C expressed in the LN ECM induces S1P production by LECs. Blockade of α9 results in lymphocyte retention in LNs (Ito et al. 2014). | |
| Spns2 | The major facilitator superfamily member Spns2 is a transporter that regulates secretion of S1P into lymph. Endothelial-specific loss of Spns2 results in reduced S1P levels in lymph and reduced egress from LNs (Mendoza et al. 2012). | |
| S1P kinase 1 and 2 | S1P kinase 1 and 2 are responsible for the production of S1P in LECs. In knockout (KO) mice, T-cell egress from LNs is impaired due to the disturbed S1P gradient (Pham et al. 2010). | |
| CLEVER-1 | Unknown ligand on T cells | Blockade of CLEVER-1 blocks T-cell adhesion to LECs forming the cortical and medullary sinuses and results in reduced entry of lymphocytes into lymphatic sinuses (Irjala et al. 2003). |
The discovery of S1P/S1PR1-mediated tissue exit in 2004 was prompted by FTY720, a natural product-derived immunosuppressive drug that at the time was in clinical development, although its mechanism of action was largely unknown. FTY720 was shown to be a structural homolog of S1P and a high-affinity functional antagonist of S1PR1 (as well as S1PR3), thereby inducing internalization of S1PR1 in lymphocytes and rendering them unresponsive to S1P (Gräler and Goetzl 2004; Matloubian et al. 2004). Treatment with FTY720 therefore results in loss of S1PR1 and lymphocyte retention in the LN. As a consequence, lymphopenia develops in lymph and blood and effector cells no longer migrate to sites of inflammation (Matloubian et al. 2004). FTY720 was approved in 2010 for the treatment of multiple sclerosis (Fingolimod/Gilenya), highlighting the relevance of lymphocyte trafficking in pathologies that involve the adaptive immune system (Chun and Brinkmann 2011).
CONCLUDING REMARKS
Technical advances like the identification of lymphatic-specific markers, the generation of lymphatic-specific KO mice or the use of photoconvertible mouse models have significantly contributed to the immense progress made over the last 20 years in our understanding of immune cell trafficking through the lymphatic vasculature. At the same time, time-lapse imaging has enabled visualizing leukocytes with single-cell resolution within LVs and paved the way to understanding the stepwise and complex dynamics of lymphatic migration in peripheral tissues and in LNs. Considering the crucial functions of the lymphatic vasculature in the induction and regulation of the immune response, it is not surprising that leukocyte migration through lymphatics is not only essential for immune protection, but also contributes to various pathologies (Oliver et al. 2020). Consequently, there is increasing interest in developing novel therapeutic approaches that modulate leukocyte trafficking in the lymphatic system in the context of immune-mediated inflammatory disorders or even in cancer (Xu et al. 2021). Most prominently, the drug Fingolimod (Novartis) was approved for multiple sclerosis in 2010 and acts by blocking the egress of T cells from LNs, ultimately trapping autoreactive T cells in this compartment. Meanwhile, several other S1P receptor modulators have been approved or entered late-stage clinical development for the treatment of multiple sclerosis and other immune-mediated inflammatory conditions (Pérez-Jeldres et al. 2021). Furthermore, novel approaches such as targeting of the sphingosine lyase or blocking S1P directly are also being investigated for therapeutic application (Vogt and Stark 2017; Pérez-Jeldres et al. 2021). Besides blocking lymphocyte egress from SLOs, modulation of leukocyte migration through afferent lymphatics could also be of therapeutic interest; for example, enhancing DC migration is expected to boost efficacy of DC-based vaccines, which are under (pre)clinical evaluation for cancer therapy (Sabado et al. 2017). Conversely, blocking DC migration has proven beneficial in murine models of corneal allograft rejection (for review, see Schönberg et al. 2020). It will be interesting to see in the future how the emerging knowledge of the cell-type-specific molecular mechanisms and functions of lymphatic migration can be translated into new therapies.
ACKNOWLEDGMENTS
The authors thank Morgan Hunter (ETH Zurich) for critical proofreading and fruitful discussion of the manuscript. C.H. gratefully acknowledges support from the Swiss National Science Foundation (Grant 310030_182528) and ETH Zurich.
Footnotes
Editors: Diane R. Bielenberg and Patricia A. D'Amore
Additional Perspectives on Angiogenesis: Biology and Pathology available at www.perspectivesinmedicine.org
REFERENCES
- Acton SE, Astarita JL, Malhotra D, Lukacs-Kornek V, Franz B, Hess PR, Jakus Z, Kuligowski M, Fletcher AL, Elpek KG, et al. 2012. Podoplanin-rich stromal networks induce dendritic cell motility via activation of the C-type lectin receptor CLEC-2. Immunity 37: 276–289. 10.1016/j.immuni.2012.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akl TJ, Nagai T, Coté GL, Gashev AA. 2011. Mesenteric lymph flow in adult and aged rats. Am J Physiol Heart Circ Physiol 301: H1828–H1840. 10.1152/ajpheart.00538.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arasa J, Collado-Diaz V, Kritikos I, Medina-Sanchez JD, Friess MC, Sigmund EC, Schineis P, Hunter MC, Tacconi C, Paterson N, et al. 2021. Upregulation of VCAM-1 in lymphatic collectors supports dendritic cell entry and rapid migration to lymph nodes in inflammation. J Exp Med 218: e20201413. 10.1084/jem.20201413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aselli G. 1627. De lactibus sive lacteis venis quarto vasorum mesaraicorum genere, novo invento. Dissertatio qua sententiæ anatomicæ multæ vel perperam receptæ, vel parù perceptæ illustrantur. Henric-Petrinis, Basileæ. [Google Scholar]
- Baeyens AAL, Schwab SR. 2020. Finding a way out: S1P signaling and immune cell migration. Ann Rev Immunol 38: 759–784. 10.1146/annurev-immunol-081519-083952 [DOI] [PubMed] [Google Scholar]
- Baluk P, Fuxe J, Hashizume H, Romano T, Lashnits E, Butz S, Vestweber D, Corada M, Molendini C, Dejana E, et al. 2007. Functionally specialized junctions between endothelial cells of lymphatic vessels. J Exp Med 204: 2349–2362. 10.1084/jem.20062596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerji S, Ni J, Wang SX, Clasper S, Su J, Tammi R, Jones M, Jackson DG. 1999. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J Cell Biol 144: 789–801. 10.1083/jcb.144.4.789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao X, Moseman EA, Saito H, Petryanik B, Thiriot A, Hatakeyama S, Ito Y, Kawashima H, Yamaguchi Y, Lowe JB, et al. 2010. Endothelial heparan sulfate controls chemokine presentation in recruitment of lymphocytes and dendritic cells to lymph nodes. Immunity 33: 817–829. 10.1016/j.immuni.2010.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastow CR, Bunting MD, Kara EE, McKenzie DR, Caon A, Devi S, Tolley L, Mueller SN, Frazer IH, Harvey N, et al. 2021. Scavenging of soluble and immobilized CCL21 by ACKR4 regulates peripheral dendritic cell emigration. Proc Natl Acad Sci 118: e2025763118. 10.1073/pnas.2025763118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazigou E, Makinen T. 2013. Flow control in our vessels: vascular valves make sure there is no way back. Cell Mol Life Sci 70: 1055–1066. 10.1007/s00018-012-1110-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazigou E, Xie S, Chen C, Weston A, Miura N, Sorokin L, Adams R, Muro AF, Sheppard D, Makinen T. 2009. Integrin-α9 is required for fibronectin matrix assembly during lymphatic valve morphogenesis. Dev Cell 17: 175–186. 10.1016/j.devcel.2009.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk DA, Swartz MA, Leu AJ, Jain RK. 1996. Transport in lymphatic capillaries. II: Microscopic velocity measurement with fluorescence photobleaching. Am J Physiol 270: H330–H337. [DOI] [PubMed] [Google Scholar]
- Bianchi R, Teijeira A, Proulx ST, Christiansen AJ, Seidel CD, Rülicke T, Mäkinen T, Hägerling R, Halin C, Detmar M. 2015. A transgenic Prox1-Cre-tdTomato reporter mouse for lymphatic vessel research. PLoS ONE 10: e0122976. 10.1371/journal.pone.0122976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun A, Worbs T, Moschovakis GL, Halle S, Hoffmann K, Bölter J, Münk A, Förster R. 2011. Afferent lymph–derived T cells and DCs use different chemokine receptor CCR7–dependent routes for entry into the lymph node and intranodal migration. Nat Immunol 12: 879–887. 10.1038/ni.2085 [DOI] [PubMed] [Google Scholar]
- Breiteneder-Geleff S, Soleiman A, Kowalski H, Horvat R, Amann G, Kriehuber E, Diem K, Weninger W, Tschachler E, Alitalo K, et al. 1999. Angiosarcomas express mixed endothelial phenotypes of blood and lymphatic capillaries: podoplanin as a specific marker for lymphatic endothelium. Am J Pathol 154: 385–394. 10.1016/S0002-9440(10)65285-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkman CC, Iwami D, Hritzo MK, Xiong Y, Ahmad S, Simon T, Hippen KL, Blazar BR, Bromberg JS. 2016. Treg engage lymphotoxin β receptor for afferent lymphatic transendothelial migration. Nat Commun 7: 12021. 10.1038/ncomms12021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromley SK, Thomas SY, Luster AD. 2005. Chemokine receptor CCR7 guides T cell exit from peripheral tissues and entry into afferent lymphatics. Nat Immunol 6: 895–901. 10.1038/ni1240 [DOI] [PubMed] [Google Scholar]
- Bromley SK, Yan S, Tomura M, Kanagawa O, Luster AD. 2013. Recirculating memory T cells are a unique subset of CD4+ T cells with a distinct phenotype and migratory pattern. J Immunol 190: 970–976. 10.4049/jimmunol.1202805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MN, Fintushel SR, Lee MH, Jennrich S, Geherin SA, Hay JB, Butcher EC, Debes GF. 2010. Chemoattractant receptors and lymphocyte egress from extralymphoid tissue: changing requirements during the course of inflammation. J Immunol 185: 4873–4882. 10.4049/jimmunol.1000676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryce SA, Wilson RAM, Tiplady EM, Asquith DL, Bromley SK, Luster AD, Graham GJ, Nibbs RJB. 2016. ACKR4 on stromal cells scavenges CCL19 to enable CCR7-dependent trafficking of APCs from inflamed skin to lymph nodes. J Immunol 196: 3341–3353. 10.4049/jimmunol.1501542 [DOI] [PubMed] [Google Scholar]
- Bujdoso R, Hopkins J, Dutia BM, Young P, McConnell I. 1989. Characterization of sheep afferent lymph dendritic cells and their role in antigen carriage. J Exp Med 170: 1285–1301. 10.1084/jem.170.4.1285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill RN, Frost H, Trnka Z. 1976. The effects of antigen on the migration of recirculating lymphocytes through single lymph nodes. J Exp Med 143: 870–888. 10.1084/jem.143.4.870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JE, Turley SJ. 2015. Stromal infrastructure of the lymph node and coordination of immunity. Trends Immunol 36: 30–39. 10.1016/j.it.2014.11.003 [DOI] [PubMed] [Google Scholar]
- Choi I, Chung HK, Ramu S, Lee HN, Kim KE, Lee S, Yoo J, Choi D, Lee YS, Aguilar B, et al. 2011. Visualization of lymphatic vessels by Prox1-promoter directed GFP reporter in a bacterial artificial chromosome-based transgenic mouse. Blood 117: 362–365. 10.1182/blood-2010-07-298562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun J, Brinkmann V. 2011. A mechanistically novel, first oral therapy for multiple sclerosis: the development of fingolimod (FTY720, Gilenya). Discov Med 12: 213–228. [PMC free article] [PubMed] [Google Scholar]
- Clark RA, Chong B, Mirchandani N, Brinster NK, Yamanaka K, Dowgiert RK, Kupper TS. 2006. The vast majority of CLA+ T cells are resident in normal skin. J Immunol 176: 4431–4439. 10.4049/jimmunol.176.7.4431 [DOI] [PubMed] [Google Scholar]
- Czeloth N, Bernhardt G, Hofmann F, Genth H, Förster R. 2005. Sphingosine-1-phosphate mediates migration of mature dendritic cells. J Immunol 175: 2960–2967. 10.4049/jimmunol.175.5.2960 [DOI] [PubMed] [Google Scholar]
- Debes GF, Arnold CN, Young AJ, Krautwald S, Lipp M, Hay JB, Butcher EC. 2005. Chemokine receptor CCR7 required for T lymphocyte exit from peripheral tissues. Nat Immunol 6: 889–894. 10.1038/ni1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Castro Pinho J, Förster R. 2021. Lymph-derived neutrophils primarily locate to the subcapsular and medullary sinuses in resting and inflamed lymph nodes. Cells 10: 1486. 10.3390/cells10061486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit D, Okuniewska M, Schwab SR. 2019. Secrets and lyase: control of sphingosine 1-phosphate distribution. Immunol Rev 289: 173–185. 10.1111/imr.12760 [DOI] [PubMed] [Google Scholar]
- Dixon JB, Greiner ST, Gashev AA, Cote GL, Moore JE, Zawieja DC. 2006. Lymph flow, shear stress, and lymphocyte velocity in rat mesenteric prenodal lymphatics. Microcirculation 13: 597–610. 10.1080/10739680600893909 [DOI] [PubMed] [Google Scholar]
- Förster R, Schubel A, Breitfeld D, Kremmer E, Renner-Müller I, Wolf E, Lipp M. 1999. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell 99: 23–33. 10.1016/S0092-8674(00)80059-8 [DOI] [PubMed] [Google Scholar]
- Förster R, Davalos-Misslitz AC, Rot A. 2008. CCR7 and its ligands: balancing immunity and tolerance. Nat Rev Immunol 8: 362–371. 10.1038/nri2297 [DOI] [PubMed] [Google Scholar]
- Förster R, Braun A, Worbs T. 2012. Lymph node homing of T cells and dendritic cells via afferent lymphatics. Trends Immunol 33: 271–280. 10.1016/j.it.2012.02.007 [DOI] [PubMed] [Google Scholar]
- Frye M, Taddei A, Dierkes C, Martinez-Corral I, Fielden M, Ortsäter H, Kazenwadel J, Calado DP, Ostergaard P, Salminen M, et al. 2018. Matrix stiffness controls lymphatic vessel formation through regulation of a GATA2-dependent transcriptional program. Nat Commun 9: 1511. 10.1038/s41467-018-03959-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard JP, Moussion C, Förster R. 2012. HEVs, lymphatics and homeostatic immune cell trafficking in lymph nodes. Nat Rev Immunol 12: 762–773. 10.1038/nri3298 [DOI] [PubMed] [Google Scholar]
- Gollmann G, Neuwirt H, Tripp CH, Mueller H, Konwalinka G, Heufler C, Romani N, Tiefenthaler M. 2008. Sphingosine-1-phosphate receptor type-1 agonism impairs blood dendritic cell chemotaxis and skin dendritic cell migration to lymph nodes under inflammatory conditions. Int Immunol 20: 911–923. 10.1093/intimm/dxn050 [DOI] [PubMed] [Google Scholar]
- Gómez D, Diehl MC, Crosby EJ, Weinkopff T, Debes GF. 2015. Effector T cell egress via afferent lymph modulates local tissue inflammation. J Immunol 195: 3531–3536. 10.4049/jimmunol.1500626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowans JL. 1957. The effect of the continuous re-infusion of lymph and lymphocytes on the output of lymphocytes from the thoracic duct of unanaesthetized rats. Br J Exp Pathol 38: 67–78. [PMC free article] [PubMed] [Google Scholar]
- Gräler MH, Goetzl EJ. 2004. The immunosuppressant FTY720 down-regulates sphingosine 1-phosphate G-protein-coupled receptors. FASEB J 18: 551–553. 10.1096/fj.03-0910fje [DOI] [PubMed] [Google Scholar]
- Grigorova IL, Panteleev M, Cyster JG. 2010. Lymph node cortical sinus organization and relationship to lymphocyte egress dynamics and antigen exposure. Proc Natl Acad Sci 107: 20447–20452. 10.1073/pnas.1009968107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hägerling R, Pollmann C, Kremer L, Andresen V, Kiefer F. 2011. Intravital two-photon microscopy of lymphatic vessel development and function using a transgenic Prox1 promoter-directed mOrange2 reporter mouse. Biochem Soc Trans 39: 1674–1681. 10.1042/BST20110722 [DOI] [PubMed] [Google Scholar]
- Halin C, Scimone ML, Bonasio R, Gauguet JM, Mempel TR, Quackenbush E, Proia RL, Mandala S, von Andrian UH. 2005. The S1P-analog FTY720 differentially modulates T-cell homing via HEV: T-cell-expressed S1P1 amplifies integrin activation in peripheral lymph nodes but not in Peyer patches. Blood 106: 1314–1322. 10.1182/blood-2004-09-3687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton HR, Bailey J, Tomura M, Brink R, Chtanova T. 2015. Microbe-dependent lymphatic migration of neutrophils modulates lymphocyte proliferation in lymph nodes. Nat Commun 6: 7139. 10.1038/ncomms8139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilligan KL, Ronchese F. 2020. Antigen presentation by dendritic cells and their instruction of CD4+ T helper cell responses. Cell Mol Immunol 17: 587–599. 10.1038/s41423-020-0465-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirosue S, Vokali E, Raghavan VR, Rincon-Restrepo M, Lund AW, Corthésy-Henrioud P, Capotosti F, Halin Winter C, Hugues S, Swartz MA. 2014. Steady-state antigen scavenging, cross-presentation, and CD8+ T cell priming: a new role for lymphatic endothelial cells. J Immunol 192: 5002–5011. 10.4049/jimmunol.1302492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho AW, Kupper TS. 2019. T cells and the skin: from protective immunity to inflammatory skin disorders. Nat Rev Immunol 19: 490–502. 10.1038/s41577-019-0162-3 [DOI] [PubMed] [Google Scholar]
- Hunter MC, Teijeira A, Montecchi R, Russo E, Runge P, Kiefer F, Halin C. 2019. Dendritic cells and T cells interact within murine afferent lymphatic capillaries. Front Immunol 10: 520. 10.3389/fimmu.2019.00520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikebuchi R, Teraguchi S, Vandenbon A, Honda T, Shand FH, Nakanishi Y, Watanabe T, Tomura M. 2016. A rare subset of skin-tropic regulatory T cells expressing Il10/Gzmb inhibits the cutaneous immune response. Sci Rep 6: 35002. 10.1038/srep35002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iolyeva M, Karaman S, Willrodt AH, Weingartner S, Vigl B, Halin C. 2013. Novel role for ALCAM in lymphatic network formation and function. FASEB J 27: 978–990. 10.1096/fj.12-217844 [DOI] [PubMed] [Google Scholar]
- Irjala H, Elima K, Johansson EL, Merinen M, Kontula K, Alanen K, Grenman R, Salmi M, Jalkanen S. 2003. The same endothelial receptor controls lymphocyte traffic both in vascular and lymphatic vessels. Eur J Immunol 33: 815–824. 10.1002/eji.200323859 [DOI] [PubMed] [Google Scholar]
- Ito K, Morimoto J, Kihara A, Matsui Y, Kurotaki D, Kanayama M, Simmons S, Ishii M, Sheppard D, Takaoka A, et al. 2014. Integrin α9 on lymphatic endothelial cells regulates lymphocyte egress. Proc Natl Acad Sci 111: 3080–3085. 10.1073/pnas.1311022111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson DG. 2019. Leucocyte trafficking via the lymphatic vasculature—mechanisms and consequences. Front Immunol 10: 471. 10.3389/fimmu.2019.00471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalkanen S, Salmi M. 2020. Lymphatic endothelial cells of the lymph node. Nat Rev Immunol 20: 566–578. 10.1038/s41577-020-0281-x [DOI] [PubMed] [Google Scholar]
- Jiang Y, Liu AH, Zhao KS. 1999. Studies on the flow and distribution of leukocytes in mesentery microcirculation of rats. World J Gastroenterol 5: 231–234. 10.3748/wjg.v5.i3.231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LA, Jackson DG. 2010. Inflammation-induced secretion of CCL21 in lymphatic endothelium is a key regulator of integrin-mediated dendritic cell transmigration. Int immunol 22: 839–849. 10.1093/intimm/dxq435 [DOI] [PubMed] [Google Scholar]
- Johnson LA, Jackson DG. 2013. The chemokine CX3CL1 promotes trafficking of dendritic cells through inflamed lymphatics. J Cell Sci 126: 5259–5270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LA, Clasper S, Holt AP, Lalor PF, Baban D, Jackson DG. 2006. An inflammation-induced mechanism for leukocyte transmigration across lymphatic vessel endothelium. J Exp Med 203: 2763–2777. 10.1084/jem.20051759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LA, Banerji S, Lawrance W, Gileadi U, Prota G, Holder KA, Roshorm YM, Hanke T, Cerundolo V, Gale NW, et al. 2017. Dendritic cells enter lymph vessels by hyaluronan-mediated docking to the endothelial receptor LYVE-1. Nat Immunol 18: 762–770. 10.1038/ni.3750 [DOI] [PubMed] [Google Scholar]
- Johnson LA, Banerji S, Lagerholm BC, Jackson DG. 2021. Dendritic cell entry to lymphatic capillaries is orchestrated by CD44 and the hyaluronan glycocalyx. Life Sci Alliance 4: e202000908. 10.26508/lsa.202000908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabashima K, Shiraishi N, Sugita K, Mori T, Onoue A, Kobayashi M, Sakabe J, Yoshiki R, Tamamura H, Fujii N, et al. 2007. CXCL12-CXCR4 engagement is required for migration of cutaneous dendritic cells. Am J Pathol 171: 1249–1257. 10.2353/ajpath.2007.070225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kähäri L, Fair-Mäkelä R, Auvinen K, Rantakari P, Jalkanen S, Ivaska J, Salmi M. 2019. Transcytosis route mediates rapid delivery of intact antibodies to draining lymph nodes. J Clin Invest 129: 3086–3102. 10.1172/JCI125740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaipainen A, Korhonen J, Mustonen T, van Hinsbergh VW, Fang GH, Dumont D, Breitman M, Alitalo K. 1995. Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proc Natl Acad Sci 92: 3566–3570. 10.1073/pnas.92.8.3566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karikoski M, Irjala H, Maksimow M, Miiluniemi M, Granfors K, Hernesniemi S, Elima K, Moldenhauer G, Schledzewski K, Kzhyshkowska J, et al. 2009. Clever-1/Stabilin-1 regulates lymphocyte migration within lymphatics and leukocyte entrance to sites of inflammation. Eur J Immunol 39: 3477–3487. 10.1002/eji.200939896 [DOI] [PubMed] [Google Scholar]
- Karuppuchamy T, Behrens EH, González-Cabrera P, Sarkisyan G, Gima L, Boyer JD, Bamias G, Jedlicka P, Veny M, Clark D, et al. 2017. Sphingosine-1-phosphate receptor-1 (S1P1) is expressed by lymphocytes, dendritic cells, and endothelium and modulated during inflammatory bowel disease. Mucosal Immunol 10: 162–171. 10.1038/mi.2016.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katakai T, Habiro K, Kinashi T. 2013. Dendritic cells regulate high-speed interstitial T cell migration in the lymph node via LFA-1/ICAM-1. J Immunol 191: 1188–1199. 10.4049/jimmunol.1300739 [DOI] [PubMed] [Google Scholar]
- Kerjaschki D, Regele HM, Moosberger I, Nagy-Bojarski K, Watschinger B, Soleiman A, Birner P, Krieger S, Hovorka A, Silberhumer G, et al. 2004. Lymphatic neoangiogenesis in human kidney transplants is associated with immunologically active lymphocytic infiltrates. J Am Soc Nephrol 15: 603–612. 10.1097/01.ASN.0000113316.52371.2E [DOI] [PubMed] [Google Scholar]
- Kienle K, Lämmermann T. 2016. Neutrophil swarming: an essential process of the neutrophil tissue response. Immunol Rev 273: 76–93. 10.1111/imr.12458 [DOI] [PubMed] [Google Scholar]
- Krummel MF, Bartumeus F, Gérard A. 2016. T cell migration, search strategies and mechanisms. Nat Rev Immunol 16: 193–201. 10.1038/nri.2015.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lämmermann T, Bader BL, Monkley SJ, Worbs T, Wedlich-Söldner R, Hirsch K, Keller M, Förster R, Critchley DR, Fässler R, et al. 2008. Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature 453: 51–55. 10.1038/nature06887 [DOI] [PubMed] [Google Scholar]
- Leak LV, Burke JF. 1966. Fine structure of the lymphatic capillary and the adjoining connective tissue area. Am J Anat 118: 785–809. 10.1002/aja.1001180308 [DOI] [PubMed] [Google Scholar]
- Ledgerwood LG, Lal G, Zhang N, Garin A, Esses SJ, Ginhoux F, Merad M, Peche H, Lira SA, Ding Y, et al. 2008. The sphingosine 1-phosphate receptor 1 causes tissue retention by inhibiting the entry of peripheral tissue T lymphocytes into afferent lymphatics. Nat Immunol 9: 42–53. 10.1038/ni1534 [DOI] [PubMed] [Google Scholar]
- Lindquist RL, Shakhar G, Dudziak D, Wardemann H, Eisenreich T, Dustin ML, Nussenzweig MC. 2004. Visualizing dendritic cell networks in vivo. Nat Immunol 5: 1243–1250. 10.1038/ni1139 [DOI] [PubMed] [Google Scholar]
- Lo CG, Xu Y, Proia RL, Cyster JG. 2005. Cyclical modulation of sphingosine-1-phosphate receptor 1 surface expression during lymphocyte recirculation and relationship to lymphoid organ transit. J Exp Med 201: 291–301. 10.1084/jem.20041509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz N, Loef EJ, Kelch ID, Verdon DJ, Black MM, Middleditch MJ, Greenwood DR, Graham ES, Brooks AE, Dunbar PR, et al. 2016. Plasmin and regulators of plasmin activity control the migratory capacity and adhesion of human T cells and dendritic cells by regulating cleavage of the chemokine CCL21. Immunol Cell Biol 94: 955–963. 10.1038/icb.2016.56 [DOI] [PubMed] [Google Scholar]
- Louie DAP, Liao S. 2019. Lymph node subcapsular sinus macrophages as the frontline of lymphatic immune defense. Front Immunol 10: 347. 10.3389/fimmu.2019.00347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas ED, Schafer JB, Matsuda J, Kraus M, Burchill MA, Tamburini BAJ. 2020. PD-L1 reverse signaling in dermal dendritic cells promotes dendritic cell migration required for skin immunity. Cell Rep 33: 108258. 10.1016/j.celrep.2020.108258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay CR, Kimpton WG, Brandon MR, Cahill RN. 1988. Lymphocyte subsets show marked differences in their distribution between blood and the afferent and efferent lymph of peripheral lymph nodes. J Exp Med 167: 1755–1765. 10.1084/jem.167.6.1755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay CR, Marston WL, Dudler L. 1990. Naive and memory T cells show distinct pathways of lymphocyte recirculation. J Exp Med 171: 801–817. 10.1084/jem.171.3.801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay CR, Marston W, Dudler L. 1992. Altered patterns of T cell migration through lymph nodes and skin following antigen challenge. Eur J Immunol 22: 2205–2210. 10.1002/eji.1830220904 [DOI] [PubMed] [Google Scholar]
- Mackay LK, Braun A, Macleod BL, Collins N, Tebartz C, Bedoui S, Carbone FR, Gebhardt T. 2015. Cutting edge: CD69 interference with sphingosine-1-phosphate receptor function regulates peripheral T cell retention. J Immunol 194: 2059–2063. 10.4049/jimmunol.1402256 [DOI] [PubMed] [Google Scholar]
- Maddaluno L, Verbrugge SE, Martinoli C, Matteoli G, Chiavelli A, Zeng Y, Williams ED, Rescigno M, Cavallaro U. 2009. The adhesion molecule L1 regulates transendothelial migration and trafficking of dendritic cells. J Exp Med 206: 623–635. 10.1084/jem.20081211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens R, Permanyer M, Werth K, Yu K, Braun A, Halle O, Halle S, Patzer GE, Bošnjak B, Kiefer F, et al. 2020. Efficient homing of T cells via afferent lymphatics requires mechanical arrest and integrin-supported chemokine guidance. Nat Commun 11: 1114. 10.1038/s41467-020-14921-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Corral I, Stanczuk L, Frye M, Ulvmar MH, Diéguez-Hurtado R, Olmeda D, Makinen T, Ortega S. 2016. Vegfr3-CreERT2 mouse, a new genetic tool for targeting the lymphatic system. Angiogenesis 19: 433–445. 10.1007/s10456-016-9505-x [DOI] [PubMed] [Google Scholar]
- Marttila-Ichihara F, Turja R, Miiluniemi M, Karikoski M, Maksimow M, Niemelä J, Martinez-Pomares L, Salmi M, Jalkanen S. 2008. Macrophage mannose receptor on lymphatics controls cell trafficking. Blood 112: 64–72. 10.1182/blood-2007-10-118984 [DOI] [PubMed] [Google Scholar]
- Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, Allende ML, Proia RL, Cyster JG. 2004. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 427: 355–360. 10.1038/nature02284 [DOI] [PubMed] [Google Scholar]
- McKimmie CS, Singh MD, Hewit K, Lopez-Franco O, Le Brocq M, Rose-John S, Lee KM, Baker AH, Wheat R, Blackbourn DJ, et al. 2013. An analysis of the function and expression of D6 on lymphatic endothelial cells. Blood 121: 3768–3777. 10.1182/blood-2012-04-425314 [DOI] [PubMed] [Google Scholar]
- McNamee EN, Masterson JC, Veny M, Collins CB, Jedlicka P, Byrne FR, Ng GY, Rivera-Nieves J. 2015. Chemokine receptor CCR7 regulates the intestinal TH1/TH17/Treg balance during Crohn's-like murine ileitis. J Leukoc Biol 97: 1011–1022. 10.1189/jlb.3HI0614-303R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza A, Bréart B, Ramos-Perez WD, Pitt LA, Gobert M, Sunkara M, Lafaille JJ, Morris AJ, Schwab SR. 2012. The transporter Spns2 is required for secretion of lymph but not plasma sphingosine-1-phosphate. Cell Rep 2: 1104–1110. 10.1016/j.celrep.2012.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoya-Zegarra JA, Russo E, Runge P, Jadhav M, Willrodt A-H, Stoma S, Nørrelykke SF, Detmar M, Halin C. 2019. Autotube: a novel software for the automated morphometric analysis of vascular networks in tissues. Angiogenesis 22: 223–236. 10.1007/s10456-018-9652-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore JE Jr, Bertram CD. 2018. Lymphatic system flows. Annu Rev Fluid Mech 50: 459–482. 10.1146/annurev-fluid-122316-045259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SN, Mackay LK. 2016. Tissue-resident memory T cells: local specialists in immune defence. Nat Rev Immunol 16: 79–89. 10.1038/nri.2015.3 [DOI] [PubMed] [Google Scholar]
- Nakamizo S, Egawa G, Tan KJ, Kabashima K. 2020. Intravital imaging of cutaneous immune responses. Cell Immunol 350: 103813. 10.1016/j.cellimm.2018.05.006 [DOI] [PubMed] [Google Scholar]
- Nakanishi Y, Ikebuchi R, Chtanova T, Kusumoto Y, Okuyama H, Moriya T, Honda T, Kabashima K, Watanabe T, Sakai Y, et al. 2017. Regulatory T cells with superior immunosuppressive capacity emigrate from the inflamed colon to draining lymph nodes. Mucosal Immunol 11: 437–448. 10.1038/mi.2017.64 [DOI] [PubMed] [Google Scholar]
- Nitschké M, Aebischer D, Abadier M, Haener S, Lucic M, Vigl B, Luche H, Fehling HJ, Biehlmaier O, Lyck R, et al. 2012. Differential requirement for ROCK in dendritic cell migration within lymphatic capillaries in steady-state and inflammation. Blood 120: 2249–2258. 10.1182/blood-2012-03-417923 [DOI] [PubMed] [Google Scholar]
- Nourshargh S, Alon R. 2014. Leukocyte migration into inflamed tissues. Immunity 41: 694–707. 10.1016/j.immuni.2014.10.008 [DOI] [PubMed] [Google Scholar]
- Ohl L, Mohaupt M, Czeloth N, Hintzen G, Kiafard Z, Zwirner J, Blankenstein T, Henning G, Förster R. 2004. CCR7 governs skin dendritic cell migration under inflammatory and steady-state conditions. Immunity 21: 279–288. 10.1016/j.immuni.2004.06.014 [DOI] [PubMed] [Google Scholar]
- Oliver G, Kipnis J, Randolph GJ, Harvey NL. 2020. The lymphatic vasculature in the 21st century: novel functional roles in homeostasis and disease. Cell 182: 270–296. 10.1016/j.cell.2020.06.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszewski WL, Grzelak I, Ziolkowska A, Engeset A. 1995. Immune cell traffic from blood through the normal human skin to lymphatics. Clin Dermatol 13: 473–483. 10.1016/0738-081X(95)00087-V [DOI] [PubMed] [Google Scholar]
- Pérez-Jeldres T, Alvarez-Lobos M, Rivera-Nieves J. 2021. Targeting sphingosine-1-phosphate signaling in immune-mediated diseases: beyond multiple sclerosis. Drugs 81: 985–1002. 10.1007/s40265-021-01528-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrova TV, Koh GY. 2018. Organ-specific lymphatic vasculature: from development to pathophysiology. J Exp Med 215: 35–49. 10.1084/jem.20171868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrova TV, Koh GY. 2020. Biological functions of lymphatic vessels. Science 369: eaax4063. 10.1126/science.aax4063 [DOI] [PubMed] [Google Scholar]
- Pflicke H, Sixt M. 2009. Preformed portals facilitate dendritic cell entry into afferent lymphatic vessels. J Exp Med 206: 2925–2935. 10.1084/jem.20091739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham TH, Okada T, Matloubian M, Lo CG, Cyster JG. 2008. S1p1 receptor signaling overrides retention mediated by Gαi-coupled receptors to promote T cell egress. Immunity 28: 122–133. 10.1016/j.immuni.2007.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham TH, Baluk P, Xu Y, Grigorova I, Bankovich AJ, Pappu R, Coughlin SR, McDonald DM, Schwab SR, Cyster JG. 2010. Lymphatic endothelial cell sphingosine kinase activity is required for lymphocyte egress and lymphatic patterning. J Exp Med 207: 17–27. 10.1084/jem.20091619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piao W, Xiong Y, Famulski K, Brinkman CC, Li L, Toney N, Wagner C, Saxena V, Simon T, Bromberg JS. 2018. Regulation of T cell afferent lymphatic migration by targeting LTβR-mediated non-classical NFκB signaling. Nat Commun 9: 3020. 10.1038/s41467-018-05412-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu C, Edwards EW, Tacke F, Angeli V, Llodrá J, Sanchez-Schmitz G, Garin A, Haque NS, Peters W, van Rooijen N, et al. 2004. Role of CCR8 and other chemokine pathways in the migration of monocyte-derived dendritic cells to lymph nodes. J Exp Med 200: 1231–1241. 10.1084/jem.20032152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph GJ, Ivanov S, Zinselmeyer BH, Scallan JP. 2017. The lymphatic system: integral roles in immunity. Annu Rev Immunol 35: 31–52. 10.1146/annurev-immunol-041015-055354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichardt P, Patzak I, Jones K, Etemire E, Gunzer M, Hogg N. 2013. A role for LFA-1 in delaying T-lymphocyte egress from lymph nodes. EMBO J 32: 829–843. 10.1038/emboj.2013.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo E, Teijeira A, Vaahtomeri K, Willrodt AH, Bloch JS, Nitschké M, Santambrogio L, Kerjaschki D, Sixt M, Halin C. 2016. Intralymphatic CCL21 promotes tissue egress of dendritic cells through afferent lymphatic vessels. Cell Rep 14: 1723–1734. 10.1016/j.celrep.2016.01.048 [DOI] [PubMed] [Google Scholar]
- Sabado RL, Balan S, Bhardwaj N. 2017. Dendritic cell-based immunotherapy. Cell Res 27: 74–95. 10.1038/cr.2016.157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F, Schaerli P, Loetscher P, Schaniel C, Lenig D, Mackay CR, Qin S, Lanzavecchia A. 1998. Rapid and coordinated switch in chemokine receptor expression during dendritic cell maturation. Eur J Immunol 28: 2760–2769. [DOI] [PubMed] [Google Scholar]
- Salmi M, Koskinen K, Henttinen T, Elima K, Jalkanen S. 2004. CLEVER-1 mediates lymphocyte transmigration through vascular and lymphatic endothelium. Blood 104: 3849–3857. 10.1182/blood-2004-01-0222 [DOI] [PubMed] [Google Scholar]
- Salmi M, Karikoski M, Elima K, Rantakari P, Jalkanen S. 2013. CD44 binds to macrophage mannose receptor on lymphatic endothelium and supports lymphocyte migration via afferent lymphatics. Circ Res 112: 1577–1582. 10.1161/CIRCRESAHA.111.300476 [DOI] [PubMed] [Google Scholar]
- Schineis P, Runge P, Halin C. 2019. Cellular traffic through afferent lymphatic vessels. Vascul Pharmacol 112: 31–41. 10.1016/j.vph.2018.08.001 [DOI] [PubMed] [Google Scholar]
- Schönberg A, Hamdorf M, Bock F. 2020. Immunomodulatory strategies targeting dendritic cells to improve corneal graft survival. J Clin Med 9: 1280. 10.3390/jcm9051280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann K, Lämmermann T, Bruckner M, Legler DF, Polleux J, Spatz JP, Schuler G, Förster R, Lutz MB, Sorokin L, et al. 2010. Immobilized chemokine fields and soluble chemokine gradients cooperatively shape migration patterns of dendritic cells. Immunity 32: 703–713. 10.1016/j.immuni.2010.04.017 [DOI] [PubMed] [Google Scholar]
- Secklehner J, Lo Celso C, Carlin LM. 2017. Intravital microscopy in historic and contemporary immunology. Immunol Cell Biol 95: 506–513. 10.1038/icb.2017.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiow LR, Rosen DB, Brdičková N, Xu Y, An J, Lanier LL, Cyster JG, Matloubian M. 2006. CD69 acts downstream of interferon-α/β to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature 440: 540–544. 10.1038/nature04606 [DOI] [PubMed] [Google Scholar]
- Skandalakis JE, Skandalakis LJ, Skandalakis PN. 2007. Anatomy of the lymphatics. Surg Oncol Clin N Am 16: 1–16. 10.1016/j.soc.2006.10.006 [DOI] [PubMed] [Google Scholar]
- Smith JB, McIntosh GH, Morris B. 1970. The traffic of cells through tissues: a study of peripheral lymph in sheep. J Anat 107: 87–100. [PMC free article] [PubMed] [Google Scholar]
- Sokol CL, Camire RB, Jones MC, Luster AD. 2018. The chemokine receptor CCR8 promotes the migration of dendritic cells into the lymph node parenchyma to initiate the allergic immune response. Immunity 49: 449–463.e6. 10.1016/j.immuni.2018.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolowski J, Jakobsen E, Johannessen JV. 1978. Cells in peripheral leg lymph of normal men. Lymphology 11: 202–207. [PubMed] [Google Scholar]
- Suami H, Scaglioni MF. 2018. Anatomy of the lymphatic system and the lymphosome concept with reference to lymphedema. Semin Plast Surg 32: 005–011. 10.1055/s-0038-1635118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz MA, Berk DA, Jain RK. 1996. Transport in lymphatic capillaries. I: Macroscopic measurements using residence time distribution theory. Am J Physiol 270: H324–H329. [DOI] [PubMed] [Google Scholar]
- Tadayon S, Dunkel J, Takeda A, Eichin D, Virtakoivu R, Elima K, Jalkanen S, Hollmén M. 2021. Lymphatic endothelial cell activation and dendritic cell transmigration is modified by genetic deletion of Clever-1. Front Immunol 12: 602122. 10.3389/fimmu.2021.602122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamatsu H, Takegahara N, Nakagawa Y, Tomura M, Taniguchi M, Friedel RH, Rayburn H, Tessier-Lavigne M, Yoshida Y, Okuno T, et al. 2010. Semaphorins guide the entry of dendritic cells into the lymphatics by activating myosin II. Nat Immunol 11: 594–600. 10.1038/ni.1885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tal O, Lim HY, Gurevich I, Milo I, Shipony Z, Ng LG, Angeli V, Shakhar G. 2011. DC mobilization from the skin requires docking to immobilized CCL21 on lymphatic endothelium and intralymphatic crawling. J Exp Med 208: 2141–2153. 10.1084/jem.20102392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teijeira A, Garasa S, Pelaez R, Azpilikueta A, Ochoa C, Marre D, Rodrigues M, Alfaro C, Auba C, Valitutti S, et al. 2013. Lymphatic endothelium forms integrin-engaging 3D structures during DC transit across inflamed lymphatic vessels. J Invest Dermatol 133: 2276–2285. 10.1038/jid.2013.152 [DOI] [PubMed] [Google Scholar]
- Teijeira A, Hunter MC, Russo E, Proulx ST, Frei T, Debes GF, Coles M, Melero I, Detmar M, Rouzaut A, et al. 2017. T cell migration from inflamed skin to draining lymph nodes requires intralymphatic crawling supported by ICAM-1/LFA-1 interactions. Cell Rep 18: 857–865. 10.1016/j.celrep.2016.12.078 [DOI] [PubMed] [Google Scholar]
- Thangada S, Khanna KM, Blaho VA, Oo ML, Im DS, Guo C, Lefrancois L, Hla T. 2010. Cell-surface residence of sphingosine 1-phosphate receptor 1 on lymphocytes determines lymphocyte egress kinetics. J Exp Med 207: 1475–1483. 10.1084/jem.20091343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tindemans I, van Schoonhoven A, KleinJan A, de Bruijn MJ, Lukkes M, van Nimwegen M, van den Branden A, Bergen IM, Corneth OB, van IJcken WFJ, et al. 2020. Notch signaling licenses allergic airway inflammation by promoting Th2 cell lymph node egress. J Clin Invest 130: 3576–3591. 10.1172/JCI128310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomura M, Yoshida N, Tanaka J, Karasawa S, Miwa Y, Miyawaki A, Kanagawa O. 2008. Monitoring cellular movement in vivo with photoconvertible fluorescence protein “Kaede” transgenic mice. Proc Natl Acad Sci 105: 10871–10876. 10.1073/pnas.0802278105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomura M, Honda T, Tanizaki H, Otsuka A, Egawa G, Tokura Y, Waldmann H, Hori S, Cyster JG, Watanabe T, et al. 2010. Activated regulatory T cells are the major T cell type emigrating from the skin during a cutaneous immune response in mice. J Clin Invest 120: 883–893. 10.1172/JCI40926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomura M, Hata A, Matsuoka S, Shand FHW, Nakanishi Y, Ikebuchi R, Ueha S, Tsutsui H, Inaba K, Matsushima K, et al. 2014. Tracking and quantification of dendritic cell migration and antigen trafficking between the skin and lymph nodes. Sci Rep 4: 6030. 10.1038/srep06030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulvmar MH, Werth K, Braun A, Kelay P, Hub E, Eller K, Chan L, Lucas B, Novitzky-Basso I, Nakamura K, et al. 2014. The atypical chemokine receptor CCRL1 shapes functional CCL21 gradients in lymph nodes. Nat Immunol 15: 623–630. 10.1038/ni.2889 [DOI] [PubMed] [Google Scholar]
- Urso K, Alvarez D, Cremasco V, Tsang K, Grauel A, Lafyatis R, von Andrian UH, Ermann J, Aliprantis AO. 2016. IL4RA on lymphatic endothelial cells promotes T cell egress during sclerodermatous graft versus host disease. JCI Insight 1: e88057. 10.1172/jci.insight.88057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaahtomeri K, Moussion C, Hauschild R, Sixt M. 2021. Shape and function of interstitial chemokine CCL21 gradients are independent of heparan sulfates produced by lymphatic endothelium. Front Immunol 12: 630002. 10.3389/fimmu.2021.630002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Broeck W, Derore A, Simoens P. 2006. Anatomy and nomenclature of murine lymph nodes: descriptive study and nomenclatory standardization in BALB/cAnNCrl mice. J Immunol Methods 312: 12–19. 10.1016/j.jim.2006.01.022 [DOI] [PubMed] [Google Scholar]
- Veiga-Fernandes H, Coles MC, Foster KE, Patel A, Williams A, Natarajan D, Barlow A, Pachnis V, Kioussis D. 2007. Tyrosine kinase receptor RET is a key regulator of Peyer's patch organogenesis. Nature 446: 547–551. 10.1038/nature05597 [DOI] [PubMed] [Google Scholar]
- Vigl B, Aebischer D, Nitschké M, Iolyeva M, Röthlin T, Antsiferova O, Halin C. 2011. Tissue inflammation modulates gene expression of lymphatic endothelial cells and dendritic cell migration in a stimulus-dependent manner. Blood 118: 205–215. 10.1182/blood-2010-12-326447 [DOI] [PubMed] [Google Scholar]
- Vogt D, Stark H. 2017. Therapeutic strategies and pharmacological tools influencing S1P signaling and metabolism. Med Res Rev 37: 3–51. 10.1002/med.21402 [DOI] [PubMed] [Google Scholar]
- von Andrian UH, Mackay CR. 2000. T-cell function and migration. Two sides of the same coin. N Engl J Med 343: 1020–1034. 10.1056/NEJM200010053431407 [DOI] [PubMed] [Google Scholar]
- Watanabe R, Gehad A, Yang C, Scott LL, Teague JE, Schlapbach C, Elco CP, Huang V, Matos TR, Kupper TS, et al. 2015. Human skin is protected by four functionally and phenotypically discrete populations of resident and recirculating memory T cells. Sci Transl Med 7: 279ra239. 10.1126/scitranslmed.3010302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber M, Hauschild R, Schwarz J, Moussion C, de Vries I, Legler DF, Luther SA, Bollenbach T, Sixt M. 2013. Interstitial dendritic cell guidance by haptotactic chemokine gradients. Science 339: 328–332. 10.1126/science.1228456 [DOI] [PubMed] [Google Scholar]
- Wee JL, Greenwood DL, Han X, Scheerlinck JP. 2011. Inflammatory cytokines IL-6 and TNF-α regulate lymphocyte trafficking through the local lymph node. Vet Immunol Immunopathol 144: 95–103. 10.1016/j.vetimm.2011.07.007 [DOI] [PubMed] [Google Scholar]
- Wigle JT, Oliver G. 1999. Prox1 function is required for the development of the murine lymphatic system. Cell 98: 769–778. 10.1016/S0092-8674(00)81511-1 [DOI] [PubMed] [Google Scholar]
- Willrodt AH, Salabarria AC, Schineis P, Ignatova D, Hunter MC, Vranova M, Golding-Ochsenbein AM, Sigmund E, Romagna A, Strassberger V, et al. 2019. ALCAM mediates DC migration through afferent lymphatics and promotes allospecific immune reactions. Front Immunol 10: 759. 10.3389/fimmu.2019.00759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong BW, Zecchin A, García-Caballero M, Carmeliet P. 2018. Emerging concepts in organ-specific lymphatic vessels and metabolic regulation of lymphatic development. Dev Cell 45: 289–301. 10.1016/j.devcel.2018.03.021 [DOI] [PubMed] [Google Scholar]
- Xiong Y, Piao W, Brinkman CC, Li L, Kulinski JM, Olivera A, Cartier A, Hla T, Hippen KL, Blazar BR, et al. 2019. CD4T cell sphingosine 1-phosphate receptor (S1PR)1 and S1PR4 and endothelial S1PR2 regulate afferent lymphatic migration. Sci Immunol 4: eaav1263. 10.1126/sciimmunol.aav1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Harris NR, Caron KM. 2021. Lymphatic vasculature: an emerging therapeutic target and drug delivery route. Ann Rev Med 72: 167–182. 10.1146/annurev-med-051419-114417 [DOI] [PubMed] [Google Scholar]
- Yanagida K, Hla T. 2017. Vascular and immunobiology of the circulatory sphingosine 1-phosphate gradient. Ann Rev Physiol 79: 67–91. 10.1146/annurev-physiol-021014-071635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yawalkar N, Hunger RE, Pichler WJ, Braathen LR, Brand CU. 2000. Human afferent lymph from normal skin contains an increased number of mainly memory/effector CD4+ T cells expressing activation, adhesion and co-stimulatory molecules. Eur J Immunol 30: 491–497. [DOI] [PubMed] [Google Scholar]
- Zawieja DC, Thangaswamy S, Wang W, Furtado R, Clement CC, Papadopoulos Z, Vigano M, Bridenbaugh EA, Zolla L, Gashev AA, et al. 2019. Lymphatic cannulation for lymph sampling and molecular delivery. J Immunol 203: 2339–2350. 10.4049/jimmunol.1900375 [DOI] [PMC free article] [PubMed] [Google Scholar]