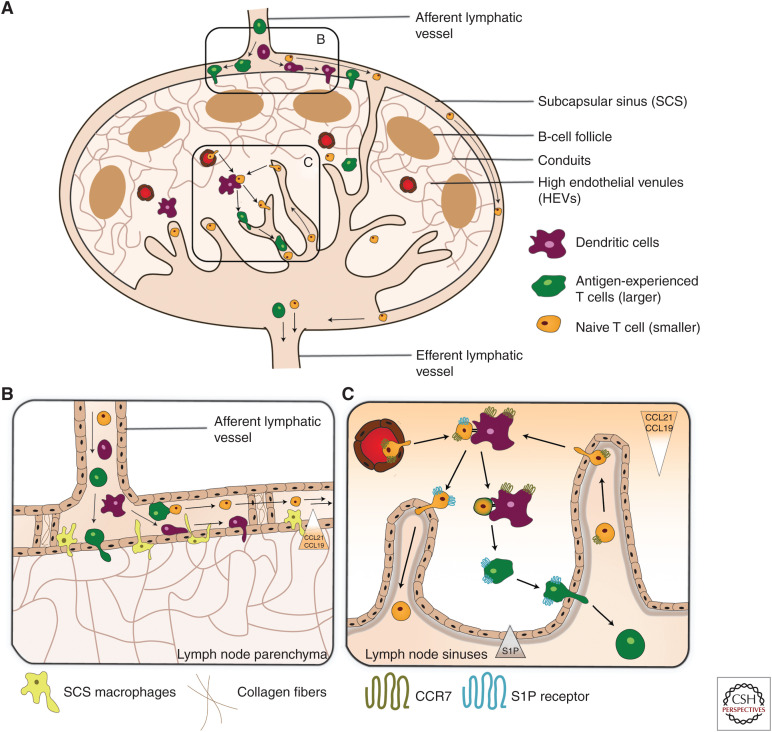
Figure 3.
Migration into draining lymph node (dLN) and exit via efferent lymphatics. Schematic illustration of (A) Leukocyte migration into and from LNs. Leukocytes enter LNs through high endothelial venules ([HEVs]; mostly T cells and B cells) or via afferent lymphatic vessels ([LVs]; mostly antigen-experienced T cells and dendritic cells [DCs]). Once in the LN parenchyma, DCs and T cells migrate into the T-cell zone and B cells into the B-cell zone, guided by chemokines. Cellular exit from LNs exclusively occurs via the lymphatic sinuses and the efferent LV. (B) Entry of lymph-borne leukocytes across the subcapsular sinus (SCS). The SCS is formed by a ceiling and floor composed of lymphatic endothelial cells (LECs) and, in the case of the floor, also SCS macrophages. The SCS lumen is interspersed with pillar-like trans-sinusoidal tissue cords composed of collagen fibers and surrounding LECs. This creates a sieve-like meshwork that only allows the free passage of smaller cells, such as naive T cells. These are passively propagated toward the medullary sinuses. Larger cells, such as DCs or activated T cells, are retained in the SCS meshwork and actively transmigrate into the LN across the SCS. Their transmigration is guided by their CCR7 receptor that senses the CCL21/CCL19 gradient directed into the T-cell zone. (C) Leukocyte entry and exit across the medullary sinuses. Small (naive) T cells arriving via the afferent LVs enter the LN by crossing the medullary sinuses. While transmigration occurs independently of CCR7, intranodal migration toward the T-cell zone is highly CCR7-dependent. Leukocyte exit is guided by the steep S1P gradient formed between the LN parenchyma and lymph. S1PR1-expressing T cells sense this gradient when in proximity of the S1P-producing LECs lining the cortical and medullary sinuses. This induces their transmigration into the lymphatic sinus system and egress from the LN via the efferent LV.