Abstract
Resveratrol is a natural polyphenol with neuroprotective function. The underlying mechanism is not well understood. Our previous studies have identified that resveratrol antagonizes cadmium (Cd) neurotoxicity via targeting PP2A/PP5-mediated Erk1/2 and JNK pathways. Here we show that resveratrol protected against Cd-poisoning also by blocking Cd-induced activation of mTORC1 and mTORC2 pathways in PC12 cells and murine primary neurons. Co-treatment with inhibitors of mTORC1 (rapamycin), mTORC1/2 (PP242), Erk1/2 (U0126) and/or JNK (SP600125), knockdown of mTOR, or disruption of mTORC1 and/or mTORC2 by silencing raptor, rictor or raptor/rictor, respectively, markedly potentiated the inhibitory effects of resveratrol on Cd-induced phosphorylation of S6K1/4E-BP1 (mTORC1 substrates), Akt (mTORC2 substrate), Erk1/2 and/or JNK/c-Jun, cleavage of caspase-3 and cell death in PC12 cells and/or primary neurons. Knockdown of S6K1 or 4E-BP1, or ectopic expression of constitutively hypophosphorylated 4E-BP1 (4E-BP1–5A) reinforced the resveratrol’s inhibition on Cd-evoked cell death, whereas ectopic expression of constitutively active S6K1 or knockdown of 4E-BP1 attenuated the resveratrol’s inhibition on Cd-induced cell death. Co-treatment with Akt inhibitor or overexpression of dominant negative Akt (dn-Akt) strengthened the resveratrol’s suppression on Cd-induced ROS, Erk1/2 activation and apoptosis, whereas overexpression of constitutively active Akt (myr-Akt) conferred high resistance to the resveratrol’s inhibitory effects in the neuronal cells. Taken together, the results indicate that resveratrol attenuates Cd-induced neuronal apoptosis partly through inhibition of mTORC1/2 pathways. Our studies highlight that resveratrol can be exploited for the prevention of Cd toxicity related to neurodegenerative diseases.
Keywords: Resveratrol, Neuroprotection, Cadmium, Poisoning, mTOR, Akt
1. Introduction
Cadmium (Cd) is a common toxic heavy metal in the environment, and oral intake is the primary way for human to expose to Cd. Numerous in vitro and in vivo studies have confirmed the neurotoxicity of Cd (Oggiano et al., 2021). The concentrations of Cd in the organs and whole body increase with age, and tend to be stable around 60 years old (Tsuchiya et al., 1976). Cd can cause oxidative stress-related toxicity in many organs and tissues of human and other mammals (Baldini et al., 2000; Mouro et al., 2021; Oldereid et al., 1993; Park et al., 2021). More seriously, Cd can penetrate the blood-brain barrier (Hedlund et al., 1979; Mostafa et al., 2019; Zhang et al., 2021a) and severely affect the function of the nervous system, leading to headache and vertigo (Bar--Sela et al., 2001), olfactory dysfunction (Schubert et al., 2021), vascular motor dysfunction (Pinheiro Junior et al., 2020), decreased balance (Poliandri et al., 2006), neurobehavioral deficit of attention (Chouit et al., 2021), psychomotor speed (Bonithon-Kopp et al., 1986) and learning disability (Ciesielski et al., 2012). Therefore, although more epidemiological evidence is needed, Cd can cause brain damage, and then lead to neurodegenerative diseases including Alzheimer’s disease (AD) (Smedman et al., 1997; Unsal et al., 2020), Parkinson’s disease (PD) (Komatsu et al., 2011; Okuda et al., 1997), Huntington’s disease (HD) (Kwakye et al., 2019; Unsal et al., 2020) and Amyotrophic Lateral Sclerosis (ALS) (Oggiano et al., 2021).
The mammalian or mechanistic target of rapamycin (mTOR), a serine/threonine (Ser/Thr) protein kinase, plays an important role in regulating cell growth, proliferation, and survival (Liu and Sabatini, 2020). mTOR functions as two complexes, mTORC1 and mTORC2 (Liu and Sabatini, 2020). mTORC1 is composed of mTOR, raptor (regulatory-associated protein of mTOR), mLST8 (also termed G protein β-subunit-like protein, GβL, a yeast homolog of LST8), PRAS40 (proline-rich Akt substrate 40 kDa) and DEPTOR (DEP-domain-containing mTOR-interacting protein), whereas mTORC2 comprises mTOR, rictor (rapamycin insensitive companion of mTOR), mLST8, mSin1 (mammalian stress-activated protein kinase-interacting protein 1), protor (protein observed with rictor) and DEPTOR (Liu and Sabatini, 2020). Ribosomal p70 S6 kinase 1 (S6K1) and eukaryotic initiation factor 4E binding protein 1 (4E-BP1) are two well-known downstream effector molecules of mTORC1, while Akt is a best-characterized substrate of mTORC2 (Cornu et al., 2013; Liu and Sabatini, 2020). Mounting evidence has demonstrated that activation or deactivation of mTORC1 and/or mTORC2 contributes to development of multiple diseases, including neurodegenerative diseases (e.g., PD, AD) (de la Torre, 2021; Jiang and Peng, 2021; Liu and Sabatini, 2020; Xu et al., 2014; Zhang et al., 2021b; Zhou et al., 2015). For example, aberrant mTOR signaling in the brain affects many pathways involved in cell viability, mitochondrial function, energy metabolism and proteostasis (Zhang et al., 2021b); PD model’s toxins, such as 6-hydroxydopamine (6-OHDA), 1-Methyl-4-phenylpyridin-1-ium (MPP+) and rotenone, can deactivate Akt/mTOR pathways leading to neuronal cell death (Jiang and Peng, 2021; Xu et al., 2014; Zhou et al., 2015). In AD mice, there exists hyperactive mTOR signaling, which damages learning and memory function, and promotes the formation of neurofibrillary tangles, a neuropathologic hallmark of AD (de la Torre, 2021). Based on the association of abnormal mTOR activity with neuronal cell malfunction, development of proper drugs that can target both mTORC1 and mTORC2 signaling pathways might be a promising strategy for prevention and treatment of neurodegenerative disorders.
Resveratrol (3,4′,5-trihydroxystilbene) is a natural polyphenolic compound mainly present in plants such as grapes, mulberry, veratrum and polygonum cuspidatum, and in several common foods like berries, peanuts and red wine (Guo et al., 2018; Oliveira et al., 2017; Su et al., 2021). Pharmacological studies have demonstrated that resveratrol possesses numerous bioactivities, including antiplatelet aggregative, anti-inflammatory, antioxidant and antiaging actions (Bastianetto et al., 2015; Guo et al., 2018; Li et al., 2012; Oliveira et al., 2017; Zhou et al., 2021). Especially, resveratrol exerts neuroprotective and therapeutic function, by effectively reducing aggravated cell damage and mitigating the progression of neurodegenerative diseases (Abdullah et al., 2020; Bastianetto et al., 2015; Guo et al., 2018; Sarroca et al., 2021). It has been shown that resveratrol inhibits mTOR signaling by activating AMP-activated protein kinase (AMPK) (Puissant et al., 2010) and enhancing the binding of DEPTOR to mTOR (Liu et al., 2010). Our group has demonstrated that Cd induces neuronal apoptosis by activating mitogen-activated protein kinases (MAPKs) and mTORC1/2 signaling network (Chen et al., 2008a, 2008b, 2011a; Xu et al., 2015; Zhang et al., 2019). Recently, we have identified that resveratrol prevents Cd-induced neuronal cell death by activating protein phosphatase 2A (PP2A) and protein phosphatase 5 (PP5), leading to suppression of extracellular signal-regulated kinases 1/2 (Erk1/2) and c-Jun N-terminal kinase (JNK) pathways (Liu et al., 2015). However, whether resveratrol protects neuronal cells from Cd-induced cell death by targeting mTORC1/2 is poorly understood.
In the study, we found that resveratrol ameliorates Cd-induced neuronal apoptosis partly through dual inhibition of mTORC1/2 signaling pathways. Our studies unveil a new mechanism by which resveratrol can prevent Cd toxicity related to neurodegenerative diseases.
2. Materials and methods
2.1. Reagents
Resveratrol, cadmium chloride, poly-d-lysine (PDL), U0126, SP600125 and protease inhibitor cocktail were purchased from Sigma (St Louis, MO, USA). Rapamycin was bought from ALEXIS (San Diego, CA, USA), whereas 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate (CM-H2DCFDA) was from MP Biomedicals (Solon, OH, USA). PP242 and Akt inhibitor X were provided by Santa Cruz Biotechnology (Santa Cruz Biotechnology, Dallas, TX, USA). Dulbecco’s modified Eagle medium (DMEM), 0.05% Trypsin-EDTA, NEURO-BASAL™ Media, and B27 Supplement were purchased from Invitrogen (Grand Island, NY, USA). Horse serum and fetal bovine serum (FBS) were supplied by Hyclone (Logan, UT, USA). Enhanced chemiluminescence solution was from Sciben Biotech Company (Nanjing, China). Other chemicals used in this work were obtained from local commercial sources and were of analytical grade.
2.2. Cell culture
Rat pheochromocytoma (PC12) cells (American Type Culture Collection, Manassas, VA, USA) were seeded in a 6-well plate or 96-well plate pre-coated with 0.2 μg/ml PDL, and cultured in antibiotic-free DMEM supplemented with 10% horse serum and 5% FBS in a humidified incubator of 5% CO2 at 37 °C. Primary cortical neurons were isolated and cultured from fetal mice at 16–18 days of gestation as described (Chen et al., 2010), and seeded in a 6-well plate or 96-well plate coated with 10 μg/mL PDL for experiments after 6 days of culture. The experiments involving animals in this study were approved by the Institutional Animal Care and Use Committee of Nanjing Normal University (Certificate NO. 200408), and were conducted in compliance with the guidelines set forth by the Guide for the Care and Use of Laboratory Animals.
2.3. Recombinant adenoviral constructs and infection of cells
Recombinant adenoviral vectors encoding β-galactosidase (Ad-LacZ), hemagglutinin (HA)-tagged constitutively hypophosphorylated 4E-BP1 (Ad-4EBP1–5A) and constitutively active S6K1 (Ad-S6K1-ca) were described previously (Liu et al., 2006, 2008). Recombinant adenoviral vectors encoding HA-tagged dominant negative Akt (Ad-dn-Akt, T308A/S473A) and constitutively active Akt (Ad-myr-Akt) were both gifted by Kenneth Walsh (Boston University, Boston, MA). The viruses were amplified, titrated and used as described (Liu et al., 2008; Shang et al., 2020). For experiments, PC12 cells were cultured in the growth medium and infected with the individual adenovirus for 24 h at 5 of multiplicity of infection (MOI = 5). Subsequently, cells were used for experiments. Ad-LacZ served as a control. Expression of HA-tagged 4E-BP1–5A, S6K1-CA, dn-Akt and myr-Akt was detected by Western blot analysis with antibodies to HA.
2.4. Lentiviral shRNA cloning and infection of cells
Lentiviral shRNAs to mTOR, raptor, rictor, raptor/rictor, S6K1, 4E-BP1 and GFP were constructed and infected as described previously (Chen et al., 2008b, 2010; Liu et al., 2006). In 5 days, cells were used for experiments.
2.5. Drugs administration and Cd treatments
Experimental treatments and timeline are shown in Fig. 1. In brief, PC12 cells, and primary neurons, or PC12 cells infected with Ad-S6K1-ca, Ad-4EBP1–5A, Ad-myr-Akt, Ad-dn-Akt or Ad-LacZ, or infected with lentiviral shRNA to mTOR, raptor, rictor, raptor/rictor, S6K1, 4E-BP1 or GFP, respectively, were seeded in a PDL-coated 6-well plate (1 × 106 cells/well for Western blotting and annexin-V-FITC/PI staining, or 1 × 105 cells/well for trypan blue exclusion) or 96-well plate (1 × 104 cells/well for caspase-3/7 activity assay), or at a density of 5 × 105 cells/well in a 6-well plate containing a PDL-coated glass coverslip per well (for TUNEL staining and ROS imaging). The next day, cells were treated with/without Cd (10 and/or 20 μM) for 4 h or 24 h following pre-incubation with/without rapamycin (200 ng/ml) for 48 h, PP242 (1 μM), U0126 (5 μM) and/or SP600125 (20 μM) for 1 h, or Akt inhibitor X (20 μM) for 1 h and/or with/without resveratrol (100 μM) for 1 h, or co-treated with/without resveratrol (100 μM)/Cd (10 and 20 μM) simultaneously for 4 h or 24 h, or pretreated with/without Cd (10 and 20 μM) for 1 h followed by exposure to resveratrol (100 μM) for 24 h, with 5 replicates of each treatment. Subsequently, analyses were conducted as described below.
Fig. 1.
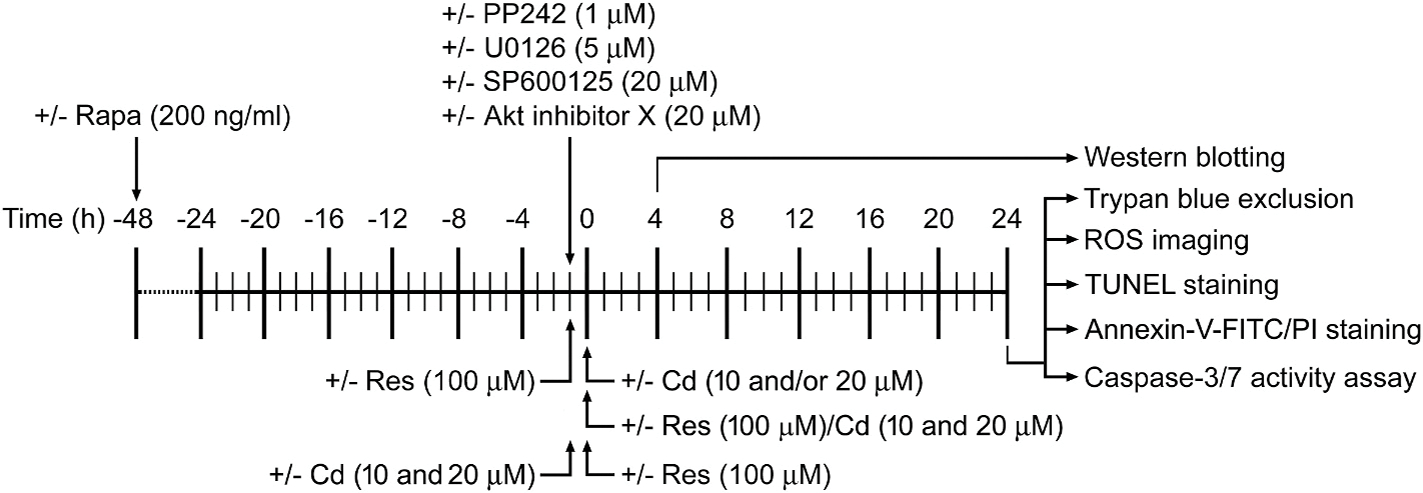
Experimental treatments and timeline in vitro. PC12 cells and primary neurons, or PC12 cells infected with Ad-S6K1-ca, Ad-4EBP1–5A, Ad-myr-Akt, Ad-dn-Akt or Ad-LacZ, or infected with lentiviral shRNA to mTOR, raptor, rictor, raptor/rictor, S6K1, 4E-BP1 or GFP, respectively, were treated with/without Cd for 4 h or 24 h following pre-incubation with/without rapamycin (Rapa) for 48 h, PP242, U0126 (5 μM) and/or SP600125 (20 μM) for 1 h, or Akt inhibitor X for 1 h and/or with/without resveratrol (Res) for 1 h, or co-treated with/without resveratrol (100 μM)/Cd (10 and 20 μM) simultaneously for 4 h or 24 h, or pretreated with/without Cd (10 and 20 μM) for 1 h followed by exposure to resveratrol (100 μM) for 24 h. Subsequently, cells were subjected to various analyses.
2.6. Live cell counting by trypan blue exclusion
The indicated cells, after treatments, were stained with 0.4% trypan blue, and live cells were monitored by counting trypan blue-negative cells.
2.7. Assay for caspase-3/7 activity
The indicated cells, after treatments, were subjected to caspase-3/7 activity assay using Caspase-Glo® 3/7 Assay Kit (Promega, Madison, WI, USA), following the instructions of the supplier.
2.8. TUNEL staining
After treatments, the indicated cells were fixed with 4% paraformaldehyde prepared in PBS for 2 h at 4 °C. The fixed cells of each slide were washed 3 times with PBS, and then incubated in the permeabilization solution (0.1% Triton 100-X, 0.1% sodium citrate) for 2 min on ice, followed by TUNEL staining according to the manufacturer’s instructions using In Situ Cell Death Detection Kit® (Roche, Mannheim, Germany). Finally, photographs for the stained samples were captured under a fluorescence microscope (200 × ) (Leica, DMi8, Wetzlar, Germany) equipped with a digital camera. For quantitative analysis of the fluorescence staining, the integral optical density (IOD) was measured by Image-Pro Plus 6.0 software (Media Cybernetics Inc., Newburyport, MA, USA).
2.9. Annexin-V-FITC/PI staining
The indicated cells, after treatments, the ratios of live, necrotic, early and late apoptotic cells were monitored by a fluorescence-activated cell sorter (FACS) flow cytometer (CytoFLEX S, Beckman, USA) using Annexin V-FITC/PI Apoptosis Detection Kit (Beyotime, Hangzhou, China), following the supplier’s instruction.
2.10. Cell ROS imaging
The indicated cells, after treatments, were loaded with an oxidant-sensitive probe, CM-H2DCFDA, followed by imaging, and then intracellular ROS fluorescence was quantitatively analyzed as described (Xu et al., 2016).
2.11. Western blot analysis
The indicated cells, after treatments, were washed with cold PBS, and then on ice, lysed in the radioimmunoprecipitation assay buffer [50 mM Tris, pH 7.2; 150 mM NaCl; 1% sodium deoxycholate; 0.1% sodium dodecyl sulfate (SDS); 1% Triton X-100; 10 mM NaF; 1 mM Na3VO4; protease inhibitor cocktail (1:1000)]. Lysates were sonicated for 10 s and centrifuged at 14,000 rpm for 10 min at 4 °C. The supernatants were collected. Protein concentration was determined by bicinchoninic acid assay with bovine serum albumin as a standard (Pierce, Rockford, IL, USA). Afterwards, Western blotting was performed as described previously (Chen et al., 2010). In brief, lysates containing equivalent amounts of protein were separated on 7–12% SDS–polyacrylamide gel and transferred to polyvinylidene difluoride membranes (Millipore, Bedford, MA, USA). Membranes were incubated with PBS containing 0.05% Tween 20 and 5% nonfat dry milk to block nonspecific binding, and then with primary antibodies against phosphorylated Akt (p-Akt) (Ser473), 4E-BP1, p-4E-BP1 (Thr70), S6 ribosomal protein, p-S6 ribosomal protein (Ser235/236), p-S6K1 (Thr389), JNK, cleaved-caspase-3 (Cell Signaling Technology, Danvers, MA, USA), Akt, p-Erk1/2 (Thr202/Tyr204), Erk2, p-c-Jun (Ser63), GSK3β, S6K1 (Santa Cruz Biotechnology), p-JNK (Thr183/Tyr185), c-Jun, p-GSK3β (Ser9) (Epitomics, Burlingame, CA, USA), raptor, rictor (Bethyl Laboratories, Montgomery, TX, USA), HA, mTOR, p-Akt (Thr308), β-tubulin (Sigma) overnight at 4 °C, respectively, followed by incubating with appropriate secondary antibodies including horseradish peroxidase-coupled goat anti-rabbit IgG, goat anti-mouse IgG, or rabbit anti-goat IgG (Pierce) overnight at 4 °C. Immunoreactive bands were visualized by using enhanced chemiluminescence solution. The blots for detected proteins were semi-quantified using NIH Image J software (National Institutes of Health, Bethesda, MD, USA).
2.12. Data and statistical analysis
Results were expressed as mean ± SEM. The normality of data was determined using Shapiro-Wilk tests. Student’s t-test for non-paired replicates was used to identify statistically significant differences between treatment means. Group variability and interaction were compared using either one-way or two-way ANOVA followed by Bonferroni’s post-tests to compare replicate means. Significance was accepted at p < 0.05, p < 0.01, p < 0.001, p < 0.0001.
3. Results
3.1. Resveratrol suppresses Cd-induced activation of Akt/mTOR pathway and apoptosis in neuronal cells
Our previous studies have demonstrated that Cd causes apoptotic cell death through activating mTOR pathway in cell cultures of PC12, SH-SY5Y cells and mouse primary neurons, as well as in mouse brain, respectively (Chen et al., 2008b, 2011b, 2014). We hypothesized that resveratrol may exert its protection against Cd neurotoxicity by blocking Cd-induced activation of mTOR signaling. For this, PC12 cells and primary neurons were pretreated with/without resveratrol (100 μM) for 1 h and then exposed to Cd (10 and 20 μM) for 4 h. Western blot analysis showed that resveratrol remarkably attenuated Cd-induced phosphorylation of S6K1 (Thr389) [one-way ANOVA: PC12 cells: p < 0.0001, F(5, 12) = 55.49; Neurons: p < 0.0001, F(5, 12) = 37.99] and 4E-BP1 (Thr70) [PC12 cells: p < 0.0001, F(5, 12) = 55.35; Neurons: p < 0.0001, F(5, 12) = 42.13] mediated by mTORC1, and Akt (Ser473) [PC12 cells: p < 0.0001, F(5, 12) = 65.49; Neurons: p < 0.0001, F(5, 12) = 29.36] mediated by mTORC2 (Fig. 2A and B). In addition, resveratrol also obviously suppressed Cd-induced phosphorylation of mTOR (Ser2488) [PC12 cells: p < 0.0001, F(5, 12) = 28.40; Neurons: p < 0.0001, F(5, 12) = 118.8], S6 (Ser235/236, a substrate of S6K1) [PC12 cells: p < 0.0001, F(5, 12) = 308.5; Neurons: p < 0.0001, F(5, 12) = 88.32] and Akt (Thr308) [PC12 cells: p < 0.0001, F(5, 12) = 24.53; Neurons: p < 0.0001, F(5, 12) = 27.08] in the cells (Fig. 2A and B). In line with our previous findings (Liu et al., 2015), resveratrol substantially decreased cleavages of caspase-3 [PC12 cells: p < 0.0001, F(5, 12) = 29.99; Neurons: p < 0.0001, F(5, 12) = 77.56] (Fig. 2A and B), the number of TUNEL-positive cells with fragmented DNA (in green) [PC12 cells: p < 0.0001, F(5, 24) = 187.7; Neurons: p < 0.0001, F(5, 24) = 162.0] (Fig. 2C and D), the ratios of apoptotic cells [PC12 cells: p < 0.0001, F(5, 12) = 135.8; Neurons: p < 0.0001, F(5, 12) = 632.1] (Fig. 2E and F), and activation of caspases 3/7 [PC12 cells: F(5, 24) = 101.4, p < 0.0001; Neurons: p < 0.0001, F(5, 24) = 61.90] (Fig. 2E) in the cells exposed to Cd, compared with the normal control. To further pinpoint the preventive and treatment effects of resveratrol on the cell death model induced by Cd, PC12 cells and primary neurons were co-treated with/without resveratrol (100 μM)/Cd (10 and 20 μM) simultaneously for 4 h or 24 h, or pretreated with/without Cd (10 and 20 μM) for 1 h followed by exposure to resveratrol (100 μM) for 24 h. Interestingly, resveratrol also powerfully blocked Cd-induced p-Akt (Ser473) [PC12 cells: p < 0.0001, F(5, 12) = 76.25; Neurons: p < 0.0001, F(5, 12) = 158.4], p-Akt (Thr308) [PC12 cells: p < 0.0001, F(5, 12) = 77.42; Neurons: p < 0.0001, F(5, 12) = 388.2], p-mTOR (Ser2488) [PC12 cells: p < 0.0001, F(5, 12) = 258.8; Neurons: p <0.0001, F(5, 12) = 234.6], p-S6K1 (Thr389) [PC12 cells: p < 0.0001, F(5, 12) = 101.1; Neurons: p < 0.0001, F(5, 12) = 148.3], p-4E-BP1 (Thr70) [PC12 cells: p < 0.0001, F(5, 12) = 126.4; Neurons: p < 0.0001, F(5, 12) = 103.0], p-S6 (Ser235/236) [PC12 cells: p < 0.0001, F(5, 12) = 35.96; Neurons: p < 0.0001, F(5, 12) = 210.7] and cleavages of caspase-3 [PC12 cells: p < 0.0001, F(5, 12) = 60.96; Neurons: p < 0.0001, F(5, 12) = 142.5] (Fig. 3A and B), and protected against Cd-induced increase in the number of TUNEL-positive cells [For Fig. 3C, PC12 cells: p < 0.0001, F(5, 18) = 48.51; Neurons: p < 0.0001, F(5, 12) = 58.12. For Fig. 3E, PC12 cells: p < 0.0001, F(5, 12) = 67.53; Neurons: p < 0.0001, F(5, 18) = 90.75.] (Fig. 3C and E) and the ratio of apoptotic cells [For Fig. 3D, PC12 cells: p < 0.0001, F(5, 12) = 228.1; Neurons: p < 0.0001, F(5, 12) = 516.5. For Fig. 3F, PC12 cells: p < 0.0001, F(5, 12) = 92.56; Neurons: p < 0.0001, F(5, 12) = 409.3] (Fig. 3D and F) in the cells. It has been described that Cd-induced activation of mTOR contributes to its neurotoxicity (Chen et al., 2008; Chen et al., 2011). Thus, the above results suggest that resveratrol mitigates Cd-induced apoptosis of neuronal cells probably by suppressing the activation of Akt/mTOR signaling.
Fig. 2.
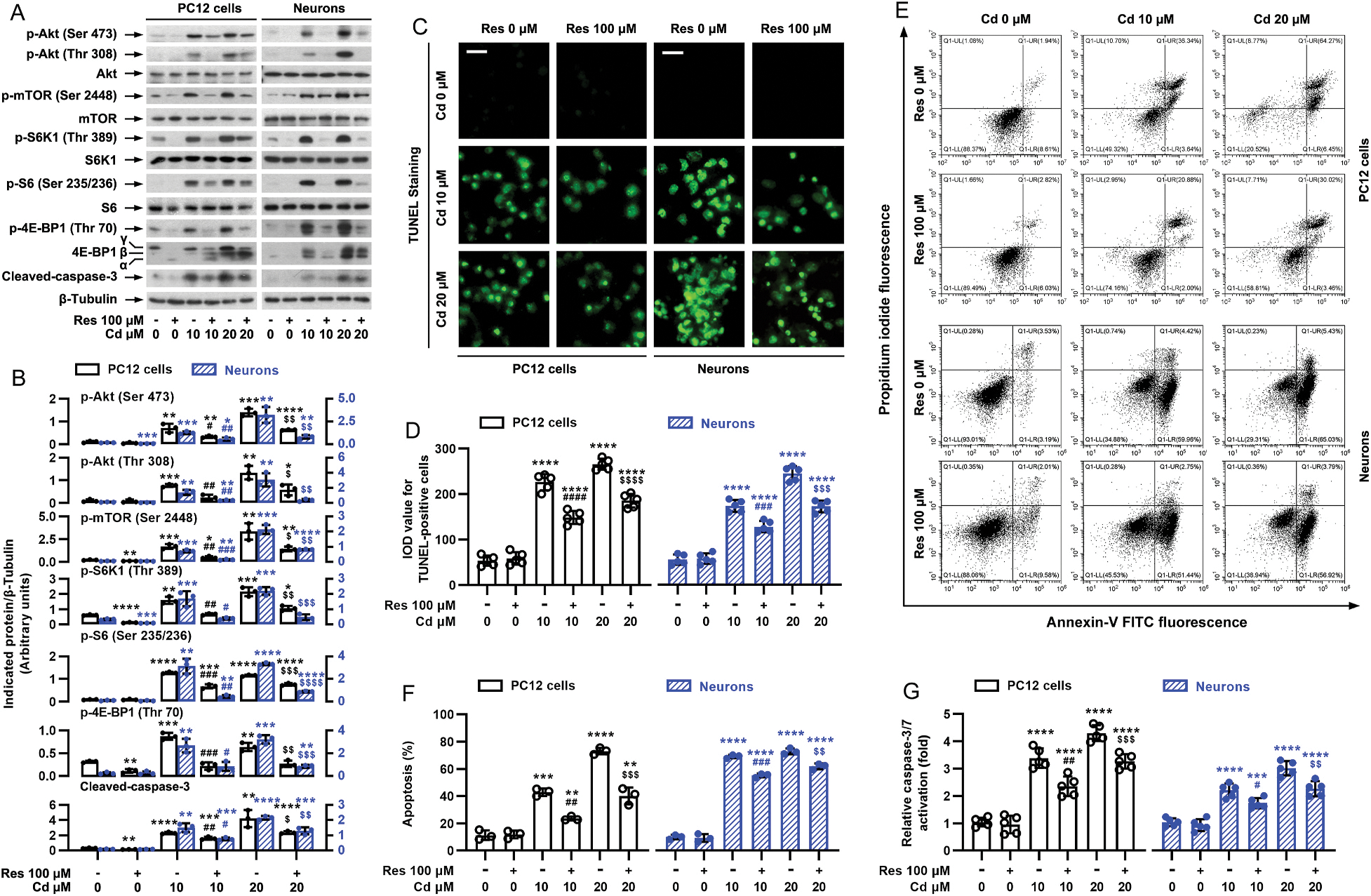
Pretreatment with resveratrol mitigates Cd-induced activation of Akt/mTOR pathway and cell apoptosis in neuronal cells. PC12 cells and primary neurons were pretreated with/without resveratrol (Res, 100 μM) for 1 h, followed by exposure to Cd (10 and 20 μM) for 4 h (for Western blotting) or 24 h (for TUNEL staining, annexin-V-FITC/PI staining and caspase-3/7 activity assay). A) Resveratrol substantially repressed Cd-induced phosphorylation of Akt, mTOR, S6K1, S6 and 4E-BP1, as well as cleavage of caspase-3 in PC12 cells and primary neurons. Total cell lysates were subjected to Western blot analysis using indicated antibodies. The blots were probed for β-tubulin as a loading control. Similar results were observed in at least five independent experiments. B) The relative densities for p-Akt (Ser473), p-Akt (Thr308), p-mTOR (Ser2448), p-S6K1 (Thr389), p-S6 (Ser235/236), p-4E-BP1 (Thr70), cleaved-caspase-3 to β-tubulin were semi-quantified using NIH image J. C) Apoptotic cells were evaluated by in situ detection of fragmented DNA (in green) using TUNEL staining. Scale bar: 20 μm. D) IOD values of TUNEL-positive cells with the fluorescence staining were quantified, showing that resveratrol markedly attenuated Cd-induced apoptosis in PC12 cells and primary neurons. E) The percentages of necrotic (Q1-UL), late apoptotic (Q1-UR), live (Q1-LL) and early apoptotic (Q1-LR) cells were determined by FACS using annexin-V-FITC/PI staining. The results from a representative experiment in PC12 cells are shown. F) Quantitative analysis of apoptotic cells by FACS assay. G) Caspase-3/7 activity was determined using Caspase-3/7 Assay Kit, showing that resveratrol significantly blocked Cd-activation of caspase-3/7 in the cells. Results are presented as mean ± SEM, n = 3–5. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, difference vs control group; #p < 0.05, ##p < 0.01, ###p < 0.001, ####p < 0.0001, difference vs 10 μM Cd group; $p < 0.05, $$p < 0.01, $$$p < 0.001, $$$$p < 0.0001, difference vs 20 μM Cd group.
Fig. 3.
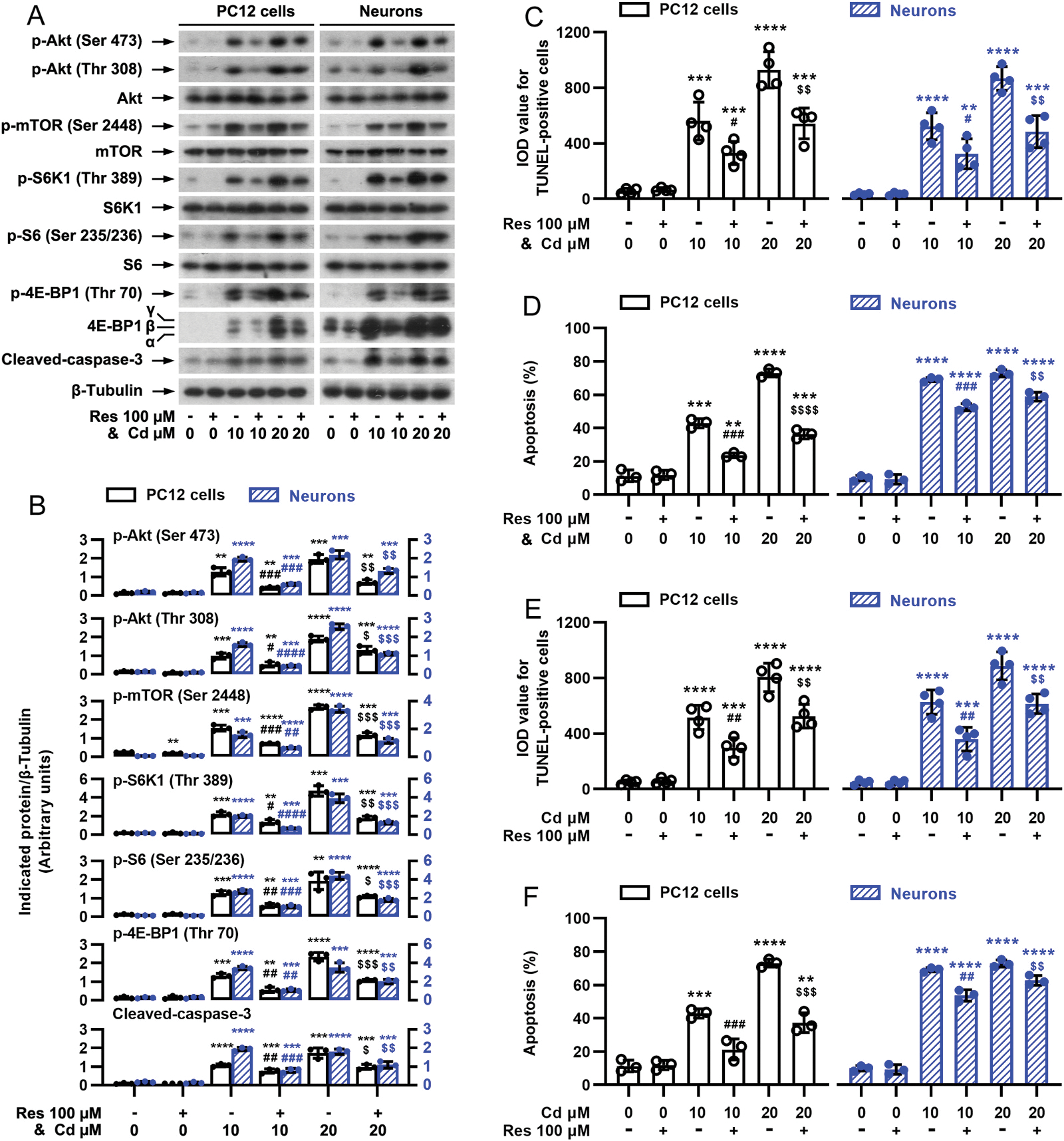
Cd-induced activation of Akt/mTOR pathway and cell apoptosis are attenuated by simultaneous or late treatment with resveratrol in neuronal cells. PC12 cells and primary neurons were co-treated with/without resveratrol (100 μM)/Cd (10 and 20 μM) simultaneously for 4 h (for Western blotting) or 24 h (for TUNEL staining and annexin-V-FITC/PI staining), or pretreated with/without Cd (10 and 20 μM) for 1 h followed by exposure to resveratrol (100 μM) for 24 h. A) Resveratrol powerfully blocked Cd-evoked p-Akt, p-mTOR, p-S6K1, p-S6, p-4E-BP1 and cleaved-caspase-3 in PC12 cells and primary neurons. Total cell lysates were subjected to Western blot analysis using indicated antibodies. The blots were probed for β-tubulin as a loading control. Similar results were observed in at least five independent experiments. B) The relative densities for p-Akt (Ser473), p-Akt (Thr308), p-mTOR (Ser2448), p-S6K1 (Thr389), p-S6 (Ser235/236), p-4E-BP1 (Thr70), cleaved-caspase-3 to β-tubulin were semi-quantified using NIH image J. C and E) IOD values of TUNEL-positive cells were evaluated by in situ detection of fragmented DNA using TUNEL staining. D and F) Quantitative analysis of apoptotic cells was determined by FACS using annexin-V-FITC/PI staining. Results are presented as mean ± SEM, n = 3–5. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, difference vs control group; #p < 0.05, ##p < 0.01, ###p < 0.001, ####p < 0.0001, difference vs 10 μM Cd group; $p < 0.05, $$p < 0.01, $$$p < 0.001, $$$$p < 0.0001, difference vs 20 μM Cd group.
3.2. Inhibition of mTOR is critical for resveratrol’s suppression of Cd-induced apoptosis in neuronal cells
To study whether resveratrol’s inhibition of Cd-induced neuronal apoptosis is related to blocking mTOR activation, PC12 cells and primary neurons were pretreated with/without rapamycin (a specific mTOR inhibitor) alone, or in combination with resveratrol. The results showed that rapamycin (200 ng/ml) or resveratrol (100 μM) alone notably restrained Cd-induced p-mTOR (Ser2488) [one-way ANOVA: PC12 cells: p < 0.0001, F(7, 16) = 211.1; Neurons: p < 0.0001, F(7, 16) = 17.0], p-S6K1 (Thr389) [PC12 cells: p < 0.0001, F(7, 16) = 38.77; Neurons: p < 0.0001, F(7, 16) = 646.1], p-S6 (Ser235/236) [PC12 cells: p < 0.0001, F(7, 16) = 270.1; Neurons: p < 0.0001, F(7, 16) = 182.9] and p-4E-BP1 (Thr70) [PC12 cells: p < 0.0001, F(7, 16) = 85.14; Neurons: p < 0.0001, F(7, 16) = 114.2], as well as the cleavage of caspase-3 [PC12 cells: p < 0.0001, F(7, 16) = 145.1; Neurons: p < 0.0001, F(7, 16) = 191.3] in the cells (Fig. 4A and B). Especially, co-treatment with resveratrol/rapamycin exhibited stronger inhibitory effects (Fig. 4A and B). Consistently, the combination of resveratrol with rapamycin also exerted more potent inhibition of Cd-evoked apoptosis than resveratrol or rapamycin alone, as evidenced by the declined number of TUNEL-positive cells [PC12 cells: p < 0.0001, F(7, 32) = 232.3; Neurons: p < 0.0001, F(7, 32) = 131.9] (Fig. 4C). Besides, knockdown of mTOR (by ~90%) in PC12 cells suppressed the basal or Cd-induced activation of mTOR signaling [two-way ANOVA: mTOR shRNA effect: p < 0.0001, F(3, 6) = 146.8; Bonferroni test for Cd effect: GFP shRNA Cd vs Cd/Res: p < 0.0001, mTOR shRNA Cd vs Cd/Res: p = 0.2701], as p-mTOR was almost non-detectable by Western blot analysis (Fig. 4D and E). Of importance, knockdown of mTOR substantially blocked Cd-induced expression of p-S6K1 [mTOR shRNA effect: p = 0.0004, F(1, 2) = 2417]/p-S6 [p = 0.0006, F(1, 2) = 1784], p-4E-BP1 [p = 0.0018, F(1, 2) = 566.0] and cleaved-caspase-3 [p = 0.0037, F(1, 2) = 271.2] in PC12 cells even without pretreatment with resveratrol (Fig. 4D and E). As expected, silencing mTOR significantly repressed Cd-induced live cell reduction [p = 0.1455, F(1, 4) = 3.255] and apoptosis [p = 0.0159, F(1, 4) = 16.14; ], and reinforced the inhibitory effect of resveratrol (Fig. 4F). Taken together, our observations underline the idea that inhibition of mTOR is critical for resveratrol’s suppression of cell apoptosis in Cd-exposed neuronal cells.
Fig. 4.
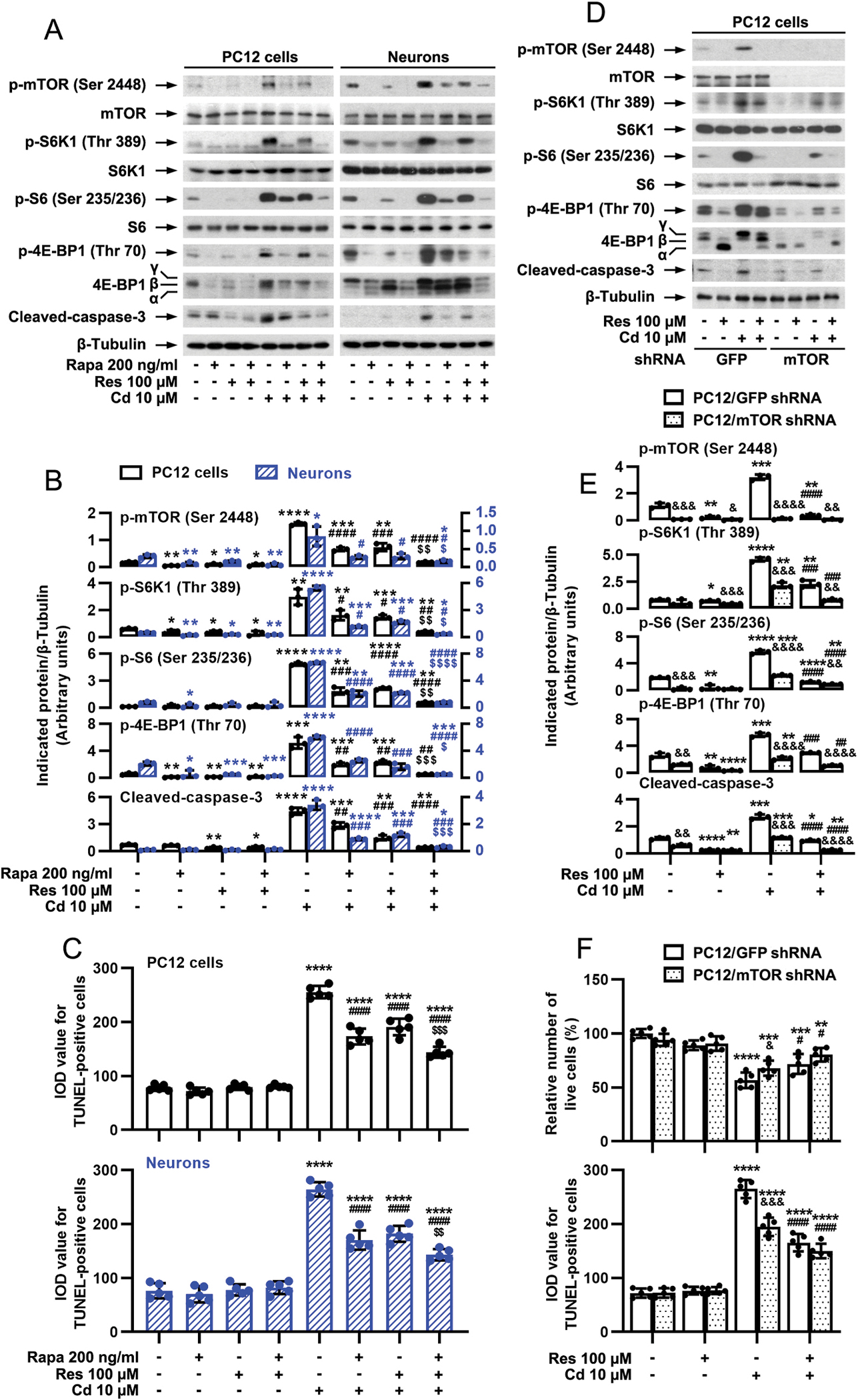
Inhibition of mTOR is critical for resveratrol’s suppression of Cd-induced apoptosis in neuronal cells. PC12 cells and primary neurons, or PC12 cells infected with lentiviral shRNAs to mTOR or GFP (as control), respectively, were pretreated with/without rapamycin (Rapa, 200 ng/ml) for 48 h and then resveratrol (Res, 100 μM) for 1 h, or pretreated with/without Res for 1 h, followed by exposure to Cd (10 μM) for 4 h (for Western blotting) or 24 h (for TUNEL staining and live cell assay). A and D) Total cell lysates were subjected to Western blotting using indicated antibodies. The blots were probed for β-tubulin as a loading control. Similar results were observed in at least five independent experiments. B and E) The relative densities for p-mTOR (Ser2448), p-S6K1 (Thr389), p-S6 (Ser235/236), p-4E-BP1 (Thr70), cleaved-caspase-3 to β-tubulin were semi-quantified using NIH image J. C and F) Apoptotic cells were evaluated by in situ detection of fragmented DNA using TUNEL staining, and live cells were detected by counting viable cells using trypan blue exclusion. Results are presented as mean ± SEM, n = 3–5. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, difference vs control group; #p < 0.05, ##p < 0.01, ###p < 0.001, ####p < 0.0001, difference vs 10 μM Cd group; $p < 0.05, $$p < 0.01, $$$p < 0.001, $$$$p < 0.0001, difference vs Cd/Res group or Cd/Rapa group; &p < 0.05, &&p < 0.01, &&&p < 0.001, &&&&p < 0.0001, mTOR shRNA group vs GFP shRNA group.
3.3. Resveratrol attenuates Cd-induced apoptosis via impeding mTORC1 and mTORC2 signaling in neuronal cells
It is well-known that mTOR functions as two complexes mTORC1 and mTORC2, which can mediate the phosphorylation of S6K/4E-BP1 and Akt, respectively (Cornu et al., 2013; Liu and Sabatini, 2020). Our current study has demonstrated that resveratrol not only dramatically hindered Cd-induced phosphorylation of S6K1/S6 and 4E-BP1, but also profoundly blocked Cd-induced phosphorylation of Akt in neuronal cells (Fig. 2A and B, 3A and B), implying that resveratrol’s protection against Cd-triggered neuronal apoptosis may be through targeting both mTORC1 and mTORC2. To determine whether this is true, first of all, PP242, an mTORC1/2 inhibitor, was employed. As predicted, PP242 powerfully inhibited both mTORC1-mediated phosphorylation of S6K1/4E-BP1 and mTORC2-mediated phosphorylation of Akt in PC12 cells and primary neurons in response to Cd exposure, and potentiated the preventive effects of resveratrol (Fig. 5A and B). Furthermore, co-treatment with PP242/resveratrol suppressed Cd-evoked cleavage of caspase-3 [one-way ANOVA: PC12 cells: p < 0.0001, F(7, 16) = 334.6; Neurons: p < 0.0001, F(7, 16) = 64.94] and apoptosis [PC12 cells: p < 0.0001, F(7, 32) = 204.0; Neurons: p < 0.0001, F(7, 32) = 155.7] more potently than PP242 or resveratrol alone (Fig. 5A–C). Our previous study has shown that U0126 (5 μM, inhibitor of MKK1/2, upstream kinases of Erk1/2) or SP600125 (20 μM, JNK inhibitor) alone substantially attenuated Cd-induced p-Erk1/2 (Thr202/Tyr204) or p-JNK (Thr183/Tyr185)/p-c-Jun (Ser63), cleaved-caspase-3 and cell death in PC12 cells and primary neurons, respectively (Liu et al., 2015). Here, we extended our study using U0126 and SP600125, showing that co-treatment with resveratrol/PP242, resveratrol/U0126 or resveratrol/SP600125 exhibited a stronger inhibitory effect on Cd-induced p-Erk1/2 (Thr202/Tyr204), p-JNK (Thr183/Tyr185), p-c-Jun (Ser63), cleavages of caspase-3, and/or apoptosis than treatment with PP242, U0126, SP600125 or resveratrol alone in PC12 cells and primary neurons (Fig. 5D–I), in agreement with our above results (Fig. 5A–C) and previous findings (Liu et al., 2015). Importantly, co-treatment with resveratrol/PP242/U0126 or resveratrol/PP242/SP600125 displayed more potent protection against Cd-induced p-Erk1/2 (Thr202/Tyr204) [PC12 cells: p < 0.0001, F(11, 24) = 462.1; Neurons: p < 0.0001, F(11, 24) = 116.8] (Fig. 5D and E), p-JNK (Thr183/Tyr185) [PC12 cells: p < 0.0001, F(11, 24) = 260.2; Neurons: p < 0.0001, F(11, 24) = 203.2] (Fig. 5G and H), p-c-Jun (Ser63) [PC12 cells: p < 0.0001, F(11, 24) = 402.2; Neurons: p < 0.0001, F(11, 24) = 565.0] (Fig. 5G and H), cleavages of caspase-3 [For Fig. 5D and E, PC12 cells: p < 0.0001, F(11, 24) = 155.5; Neurons: p < 0.0001, F(11, 24) = 266.6. For Fig. 5G and H, PC12 cells: p < 0.0001, F(11, 24) = 210.2; Neurons: p < 0.0001, F(11, 24) = 310.0], and increase in the number of TUNEL-positive cells [For Fig. 5F, PC12 cells: p < 0.0001, F(11, 48) = 230.4; Neurons: p < 0.0001, F(11, 48) = 587. For Fig. 5I, PC12 cells: p < 0.0001, F(11, 48) = 251.8; Neurons: p < 0.0001, F(11, 48) = 320.3] than resveratrol/PP242, resveratrol/U0126, or resveratrol/SP600125 in the cells (Fig. 5D–I). These data suggest that resveratrol’s neuroprotection from Cd toxicity, is at least partially by suppressing the activation of both mTORC1 and mTORC2, and hindering the activation of Erk1/2 and/or JNK pathways.
Fig. 5.
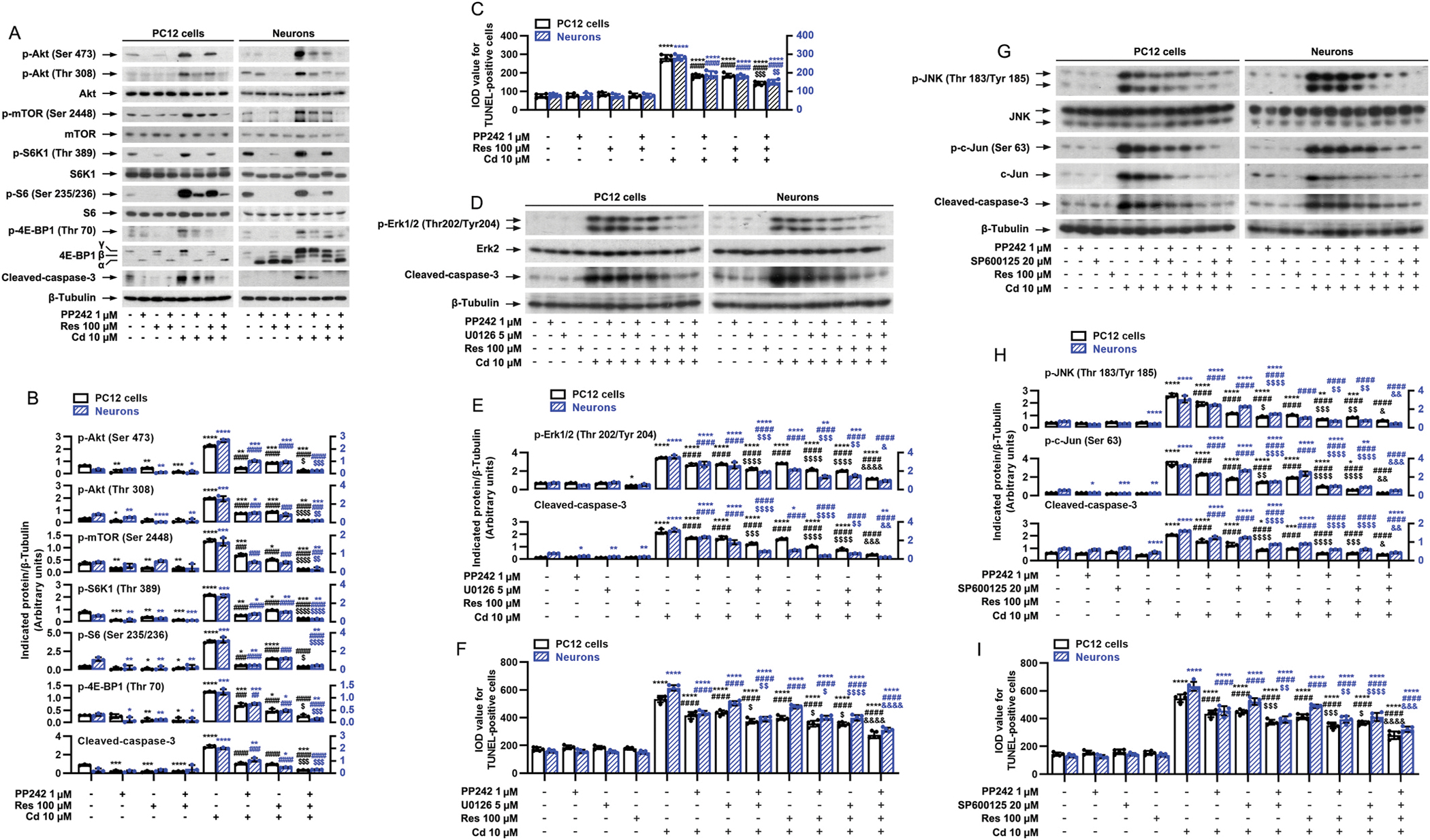
Inhibition of mTORC1/2, Erk1/2 or JNK by PP242, U0126 and/or SP600125 strengthens resveratrol’s protection against Cd-induced apoptosis in neuronal cells. PC12 cells and primary neurons were pretreated with/without PP242 (1 μM), U0126 (5 μM) and/or SP600125 (20 μM) for 1 h and then resveratrol (Res, 100 μM) for 1 h, followed by exposure to Cd (10 μM) for 4 h (for Western blotting) or 24 h (for TUNEL staining). A, D and G) Total cell lysates were subjected to Western blotting using indicated antibodies. The blots were probed for β-tubulin as a loading control. Similar results were observed in at least five independent experiments. B, E and H) The relative densities for p-Akt (Ser473), p-Akt (Thr308), p-mTOR (Ser2448), p-S6K1 (Thr389), p-S6 (Ser235/236), p-4E-BP1 (Thr70), p-Erk1/2 (Thr202/Tyr204), p-JNK (Thr183/Tyr185), p-c-Jun (Ser63), cleaved-caspase-3 to β-tubulin were semi-quantified using NIH image J. C, F and I) Apoptotic cells were evaluated by in situ detection of fragmented DNA using TUNEL staining. Results are presented as mean ± SEM, n = 3–5. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, difference vs control group; #p < 0.05, ##p < 0.01, ###p < 0.001, ####p < 0.0001, difference vs 10 μM Cd group; $p < 0.05, $$p < 0.01, $$$p < 0.001, $$$$p < 0.0001, difference vs Cd/Res group, Cd/PP242 group, Cd/U0126 group or Cd/SP600125 group; &p < 0.05, &&p < 0.01, &&&p < 0.001, &&&&p < 0.0001, difference vs Cd/Res/PP242 group, Cd/Res/U0126 group or Cd/Res/SP600125 group.
Raptor and rictor are respectively key components of mTORC1 and mTORC2, which are essential for their distinct functions (Cornu et al., 2013; Liu and Sabatini, 2020). To determine the role of mTORC1 and mTORC2 in the protection of resveratrol against Cd’s neurotoxicity, mTORC1 and mTORC2 were disrupted by silencing raptor and rictor, respectively. For this, PC12 cells, infected with lentiviral shRNAs to raptor, rictor, raptor/rictor or GFP (as control), respectively, were pretreated with/without resveratrol (100 μM) for 1 h, followed by exposure to Cd (10 μM) for 4 h or 24 h. Western blot analysis revealed that lentiviral shRNAs to raptor, rictor or raptor/rictor, but not GFP, downregulated raptor, rictor or raptor/rictor protein expression by ~90% in the cells (Fig. 6A and B). Knockdown of raptor powerfully blocked Cd-induced phosphorylation of S6K1/4E-BP1, and knockdown of rictor dramatically impeded Cd-induced phosphorylation of Akt (Ser 473), whereas double knockdown of raptor/rictor exhibited a more potent inhibitory effect on Cd-induced phosphorylation of Akt [two-way ANOVA: p = 0.0005, F(1.335, 2.711) = 377.5], S6K1 [p = 0.0195, F(1.026, 2.052) = 46.57] and 4E-BP1 [p = 0.0036, F(1.048, 2.097) = 226.5] than the single knockdown of raptor or rictor (Fig. 6A and B). Of importance, pretreatment with resveratrol for 1 h not only substantially inhibited Cd-induced phosphorylation of S6K1/4E-BP1, but also markedly repressed Cd-induced phosphorylation of Akt in PC12 cells infected with lentiviral shRNA to raptor, rictor, raptor/rictor or GFP (Fig. 6A and B). Of interest, depleting raptor or rictor potentiated the inhibitory effect of resveratrol on Cd-induced cleavage of caspase-3, live cell reduction [p = 0.0023, F(2.262, 9.048) = 12.12] and apoptosis [p < 0.0001, F(2.134, 8.536) = 44.32] (Fig. 6A–D). Furthermore, double knockdown of raptor/rictor showed more potent effect than single knockdown of raptor or rictor (Fig. 6A–D). These results further suggest that resveratrol attenuates Cd-induced apoptosis via impeding mTORC1 and mTORC2 signaling pathways in neuronal cells.
Fig. 6.
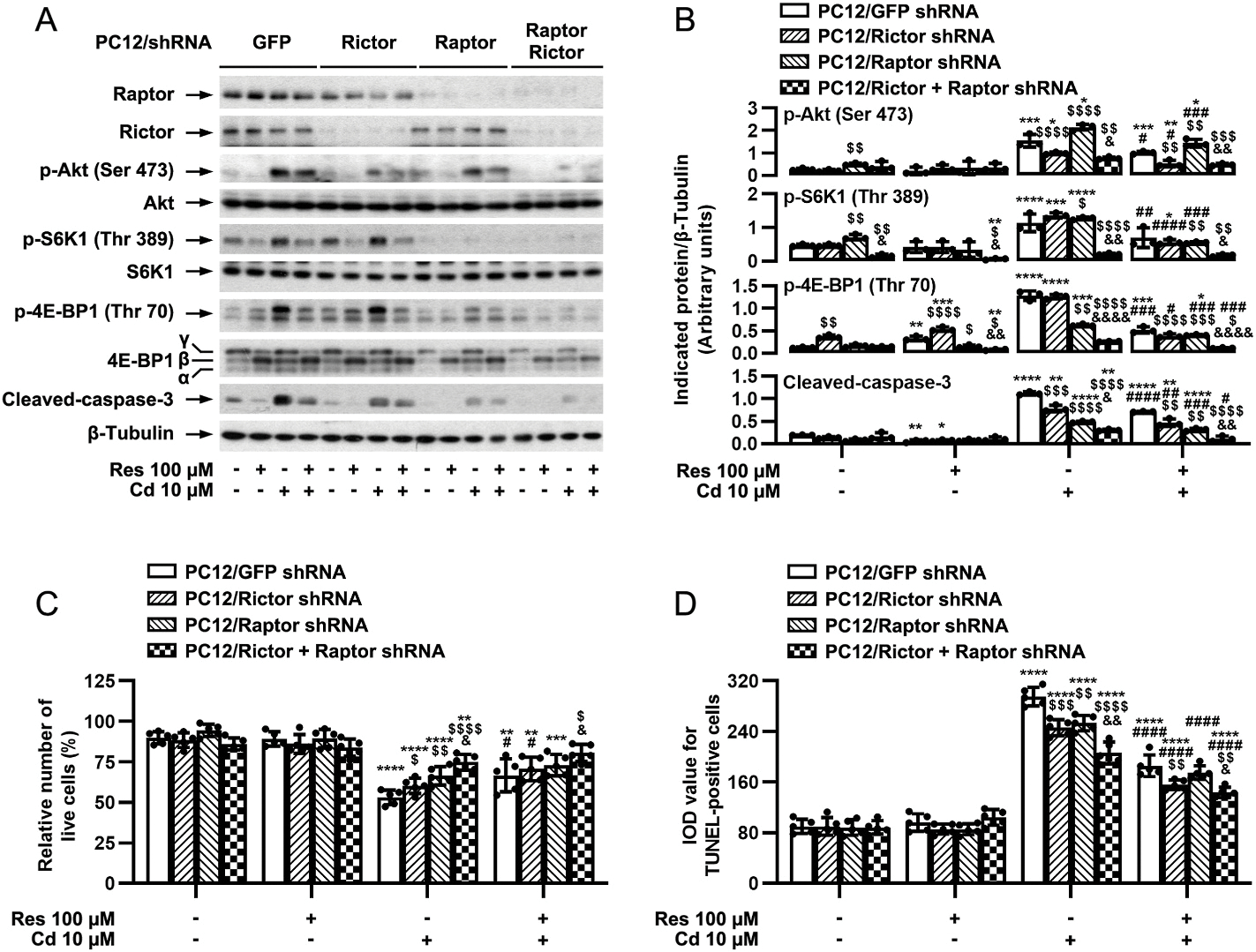
Disruption of mTORC1 and/or mTORC2 potentiates resveratrol’s prevention from Cd-induced apoptosis in neuronal cells. PC12 cells, infected with lentiviral shRNAs to raptor, rictor, raptor/rictor or GFP (as control), were pretreated with/without resveratrol (Res, 100 μM) for 1 h, followed by exposure to Cd (10 μM) for 4 h (for Western blotting) or 24 h (for live cell assay and TUNEL staining). A) Total cell lysates were subjected to Western blotting using indicated antibodies. The blots were probed for β-tubulin as a loading control. Similar results were observed in at least five independent experiments. B) The blots for p-Akt (Ser473), p-S6K1 (Thr389), p-4E-BP1 (Thr70), cleaved-caspase-3 to β-tubulin were semi-quantified using NIH image J. C and D) Live and apoptotic cells were evaluated by counting viable cells using trypan blue exclusion (C) and by in situ detection of fragmented DNA using TUNEL staining (D), respectively. Results are presented as mean ± SEM, n = 3–5. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, difference vs control group; #p < 0.05, ##p < 0.01, ###p < 0.001, ####p < 0.0001, difference vs 10 μM Cd group; $p < 0.05, $$p < 0.01, $$$p < 0.001, $$$$p < 0.0001, Raptor shRNA group, Rictor shRNA group or Raptor/Rictor shRNA group vs GFP shRNA group; &p < 0.05, &&p < 0.01, &&&p < 0.001, &&&&p < 0.0001, Raptor/Rictor shRNA group vs Raptor shRNA group or Rictor shRNA group.
3.4. mTORC1-mediated S6K1 and 4E-BP1 pathways are necessary for resveratrol’s rescue from Cd-induced apoptosis in neuronal cells
S6K1 and 4E-BP1 are the two key downstream effector molecules of mTORC1 (Cornu et al., 2013; Liu and Sabatini, 2020). To substantiate that the protection of against Cd’s neurotoxicity is by targeting mTORC1 signaling, firstly, we studied the role of S6K1 in this process. For this, on the one hand, S6K1 was knocked down in PC12 cells (Fig. 7A). Silencing S6K1 profoundly reduced the basal and Cd-induced phosphorylation of S6K1 [two-way ANOVA: p = 0.0004, F(1, 2) = 2246]/S6 [p = 0.0012, F(1, 2) = 799.0] and cleavage of caspase-3 [p = 0.0022, F(1, 2) = 460.0] (Fig. 7A and B). By live cell assay and TUNEL staining, we observed that silencing S6K1 alone partially prevented Cd-induced live cell reduction [p = 0.0563, F(1, 4) = 7.082] and apoptosis [p = 0.0005, F(1, 4) = 109.9], which was enhanced by addition of resveratrol (Fig. 7C). On the other hand, constitutively active S6K1 (S6K1-ca) was expressed in PC12 cells. As expected, a high level of HA-tagged S6K1-ca was seen in Ad-S6K1-ca-infected cells, but not in Ad-LacZ-infected cells (as control); and cells expressing S6K1-ca had a robust phosphorylation of S6K1 [p = 0.0009, F(1, 2) = 1100] (Fig. 7D and E). Cd treatment failed to further enhance the phosphorylation of S6K1 in the cells infected with Ad-S6K1-ca (Fig. 7D and E). Ectopic expression of S6K1-ca conferred resistance to PP242 or resveratrol inhibition of the basal and Cd-induced p-Akt, p-S6K1, p-S6, cleavage of caspase-3, live cell reduction [live cells’ effect: p = 0.0001, F(1, 4) = 196.9], and apoptosis [TUNEL-positive cells’ effect: p = 0.0007, F(1, 4) = 91.99] in the cells (Fig. 7D–F). The findings support that resveratrol inhibits Cd-induced neuronal apoptosis in part by suppressing mTORC1-mediated S6K1 pathway.
Fig. 7.
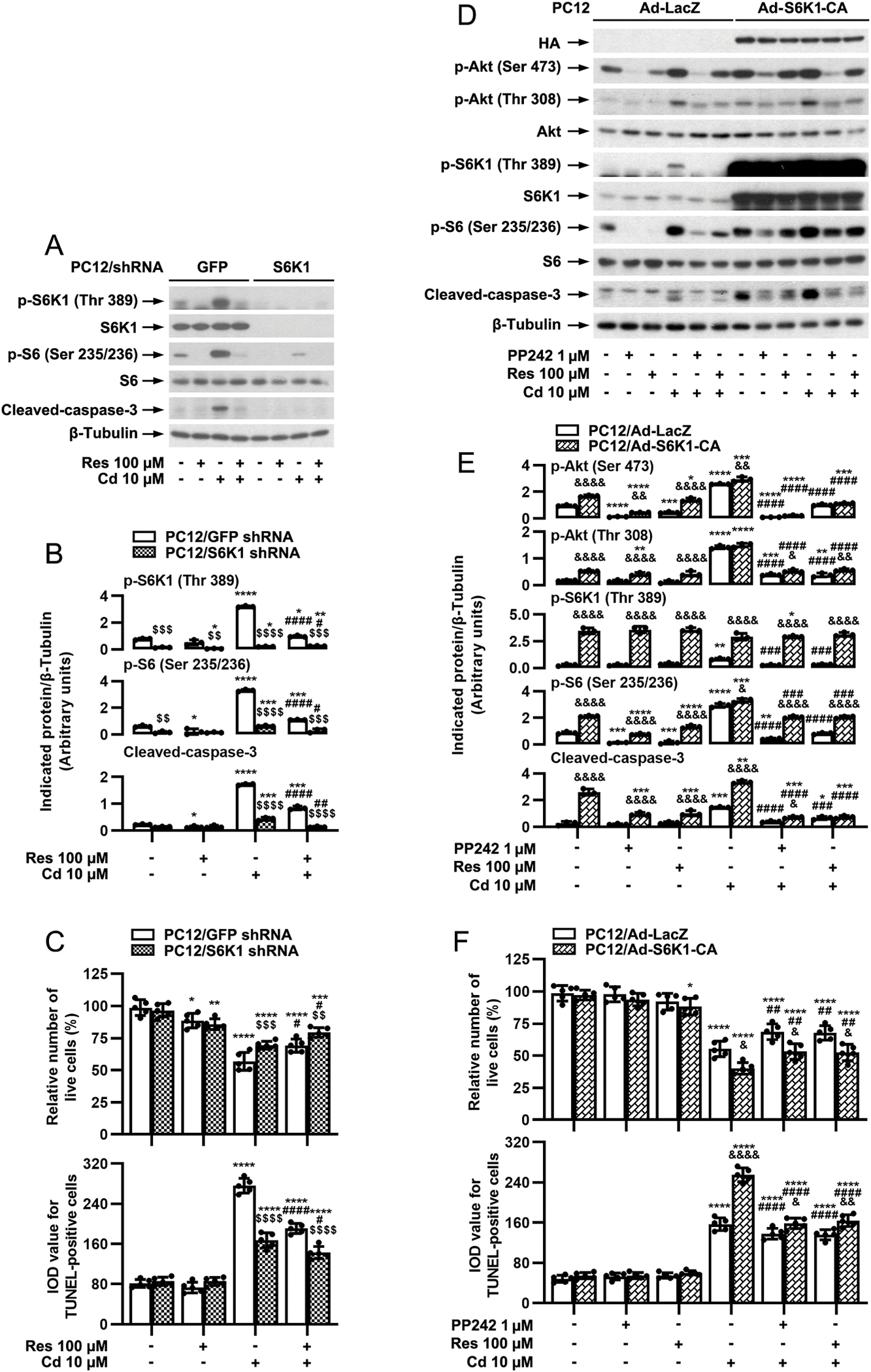
mTORC1-mediated S6K1 pathway is involved in resveratrol’s protection against Cd-induced apoptosis in neuronal cells. PC12 cells, infected with lentiviral shRNA to S6K1 or GFP (as control), or with Ad-S6K1-ca or Ad-LacZ, were pretreated with/without resveratrol (Res, 100 μM) for 1 h, or pretreated with/without PP242 (1 μM) for 1 h and then resveratrol Res for 1 h, followed by exposure to Cd (10 μM) for 4 h (for Western blotting) or 24 h (for live cell assay and TUNEL staining). A and D) Total cell lysates were subjected to Western blotting using indicated antibodies. The blots were probed for β-tubulin as a loading control. Similar results were observed in at least five independent experiments. B and E) The relative densities for p-Akt (Ser473), p-Akt (Thr308), p-S6K1 (Thr389), p-S6 (Ser235/236), cleaved-caspase-3 to β-tubulin were semi-quantified using NIH image J. C and F) Live cells were detected by counting viable cells using trypan blue exclusion, and apoptotic cells were evaluated by in situ detection of fragmented DNA using TUNEL staining. Results are presented as mean ± SEM, n = 3–5. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, difference vs control group; #p < 0.05, ##p < 0.01, ###p < 0.001, ####p < 0.0001, difference vs 10 μM Cd group; $p < 0.05, $$p < 0.01, $$$p < 0.001, $$$$p < 0.0001, S6K1 shRNA group vs GFP shRNA group; &p < 0.05, &&p < 0.01, &&&p < 0.001, &&&&p < 0.0001, Ad-S6K1-ca group vs Ad-LacZ group.
Meanwhile, we also studied the role of 4E-BP1/eIF4E pathway in resveratrol’s neuroprotection from Cd-poisoning. To this end, firstly, PC12 cells were infected with a recombinant adenovirus encoding HA-tagged constitutively hypophosphorylated 4E-BP1 mutants where Thr36, Thr45, Ser64, Thr69 andSer82 are replaced by Ala residues (Ad-4EBP1–5A), which can tightly bind to and sequester eIF4E in cells (Mothe-Satney et al., 2000). As shown in Fig. 8A, HA-tagged 4E-BP1 and higher levels of 4E-BP1 were detected in Ad-4EBP1–5A-infected PC12 cells. Resveratrol treatment failed to alter the mobility of 4E-BP1–5A (Fig. 8A). Interestingly, expression of 4E-BP1–5A profoundly reinforced the inhibitory effects of resveratrol on Cd-induced caspase-3 cleavage [two-way ANOVA: p = 0.0026, F(1, 2) = 386.4; ], live cell reduction [p = 0.4873, F(1, 4) = 0.5842] and apoptosis [p = 0.0014, F(1, 4) = 61.5] (Fig. 8A–E). Next, 4E-BP1 was silenced in PC12 cells. Similarly, knockdown of 4E-BP1 powerfully potentiated resveratrol inhibition of the basal and Cd-induced p-4E-BP1 cleavage of caspase-3 [p < 0.0001, F(1, 2) = 15056], live cell reduction [p < 0.0001, F(1, 11) = 494.6 and apoptosis [p = 0.0007, F(1, 4) = 88.38] in the cells (Fig. 8F–H) as well. These observations indicate that resveratrol suppresses Cd-induced neuronal cell death also partly by targeting mTORC1-mediated 4E-BP1/eIF4E pathway. Collectively, both S6K1 and 4E-BP1 pathways mediated by mTORC1 are involved in resveratrol’s protection from Cd-induced apoptosis in neuronal cells.
Fig. 8.
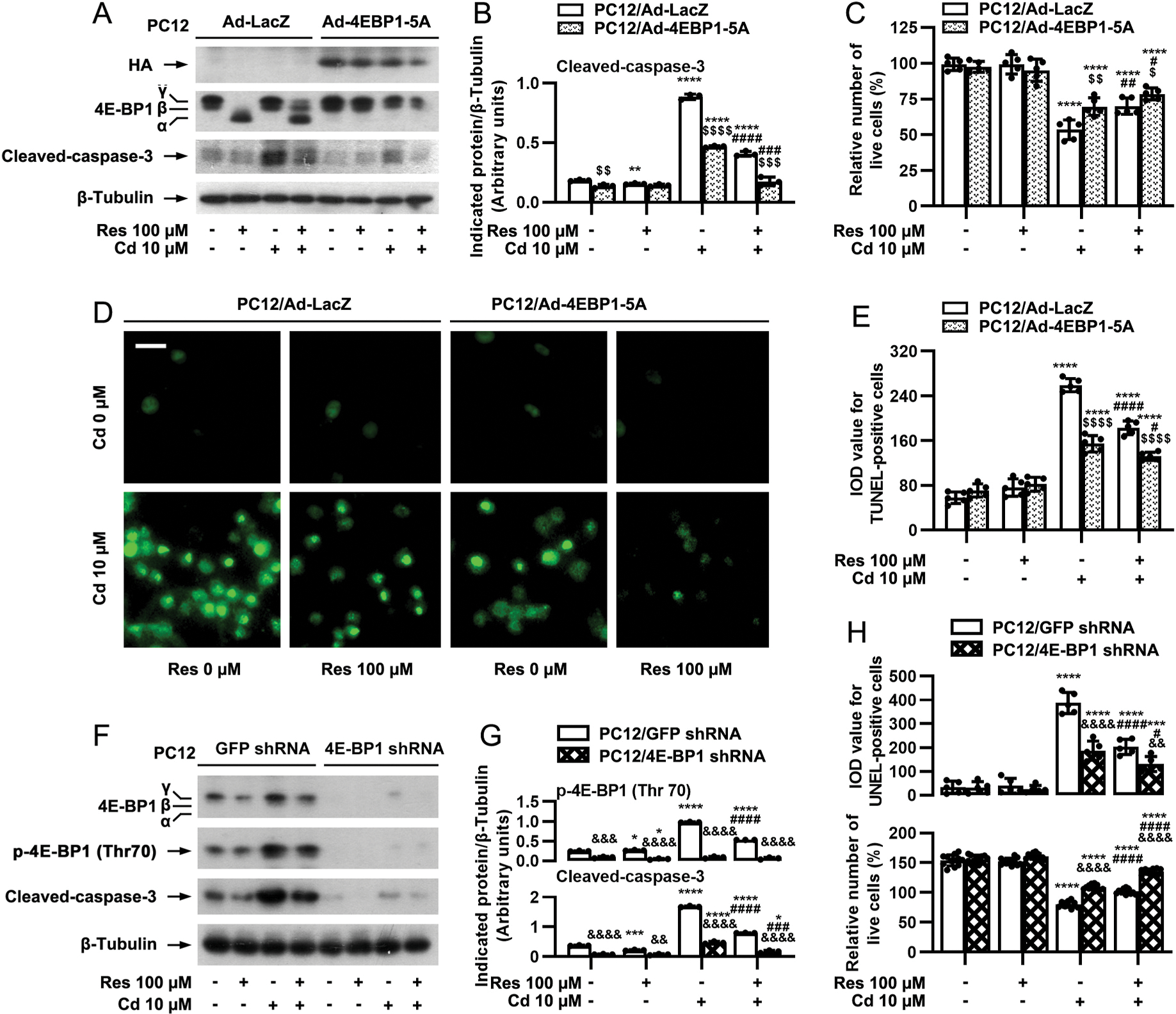
mTORC1-mediated 4E-BP1 pathway participates in resveratrol’s rescue from Cd-induced apoptosis in neuronal cells. PC12 cells, infected with Ad-4EBP1–5A or Ad-LacZ (as control), or with lentiviral shRNA to 4E-BP1 or GFP (as control), were pretreated with/without resveratrol (Res, 100 μM) for 1 h, followed by exposure to Cd (10 μM) for 4 h (for Western blotting) or 24 h (for live cell assay and TUNEL staining). A and F) Total cell lysates were subjected to Western blot analysis using indicated antibodies. The blots were probed for β-tubulin as a loading control. Similar results were observed in at least five independent experiments. B and G) The relative densities for p-4E-BP1 (Thr70), cleaved-caspase-3 to β-tubulin were semi-quantified using NIH image J. C, D, E and H) Live and apoptotic cells were evaluated by counting viable cells using trypan blue exclusion and by in situ detection of fragmented DNA using TUNEL staining, respectively. Results are presented as mean ± SEM, n = 3–5. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, difference vs control group; #p < 0.05, ##p < 0.01, ###p < 0.001, ####p < 0.0001, difference vs 10 μM Cd group; $p < 0.05, $$p < 0.01, $$$p < 0.001, $$$$p < 0.0001, Ad-4EBP1–5A group vs Ad- LacZ group; &p < 0.05, &&p < 0.01, &&&p < 0.001, &&&&p < 0.0001, 4E-BP1 shRNA group vs GFP shRNA group.
3.5. mTORC2-mediated Akt pathway contributes to resveratrol’s inhibition of Cd-induced Erk1/2 activation, ROS generation and apoptosis in neuronal cells
We have demonstrated that mTORC2 participates in resveratrol’s inhibition of Cd-induced neuronal apoptosis (Figs. 5 and 6). Since mTORC2 directly phosphorylates Akt on Ser473 (Cornu et al., 2013; Liu and Sabatini, 2020), we therefore studied whether mTORC2-mediated Akt pathway is involved in resveratrol’s inhibition of Cd-induced neuronal apoptosis. To answer this question, Akt inhibitor X, a selective Akt inhibitor, was employed. We observed that pretreatment with Akt inhibitor X or resveratrol significantly repressed the basal and Cd-induced p-Akt (Ser473) [one-way ANOVA: PC12 cells: p < 0.0001, F(7, 16) = 152.0; Neurons: p < 0.0001, F(7, 16) = 173.4], p-Akt (Thr308) [PC12 cells: p < 0.0001, F(7, 16) = 307.2; Neurons: p < 0.0001, F(7, 16) = 386.5] and its substrate p-GSK3β (Ser9) [PC12 cells: p < 0.0001, F(7, 16) = 551.5; Neurons: p < 0.0001, F(7, 16) = 188.7] in PC12 cells and primary neurons (Fig. 9A and B). In addition, similar effects on the basal or Cd-induced p-S6K1 (Thr389) [PC12 cells: p < 0.0001, F(7, 16) = 539.1; Neurons: p < 0.0001, F(7, 16) = 148.5], p-S6 (Ser235/236) [PC12 cells: p < 0.0001, F(7, 16) = 155.9; Neurons: p < 0.0001, F(7, 16) = 148.0] and p-4E-BP1 (Thr70) [PC12 cells: p < 0.0001, F(7, 16) = 31.34,; Neurons: p < 0.0001, F(7, 16) = 21.53] and p-Erk1/2 (Thr202/Tyr204) [PC12 cells: p < 0.0001, F(7, 16) = 241.5,; Neurons: p < 0.0001, F(7, 16) = 1334] were also seen in the cells treated with Akt inhibitor X or resveratrol (Fig. 9A and B). Especially, co-treatment with Akt inhibitor X/resveratrol showed stronger inhibitory effects (Fig. 9A and B). Consistently, co-treatment with Akt inhibitor X/resveratrol also inhibited Cd-evoked expression of cleaved-caspase-3 [PC12 cells: p < 0.0001, F(7, 16) = 427.8; Neurons: p < 0.0001, F(7, 16) = 176.4], generation of ROS [PC12 cells: p < 0.0001, F(7, 32) = 154.9; Neurons: p < 0.0001, F(7, 32) = 175.2] and number of TUNEL-positive cells [PC12 cells: p < 0.0001, F(7, 32) = 154.7; Neurons: p < 0.0001, F(7, 32) = 188.0,] more potently than Akt inhibitor X or resveratrol alone (Fig. 9A–E), suggesting that mTORC2-mediated Akt pathway may be associated with resveratrol’s inhibition of Cd-induced Erk1/2 activation, ROS generation and neuronal apoptosis as well.
Fig. 9.
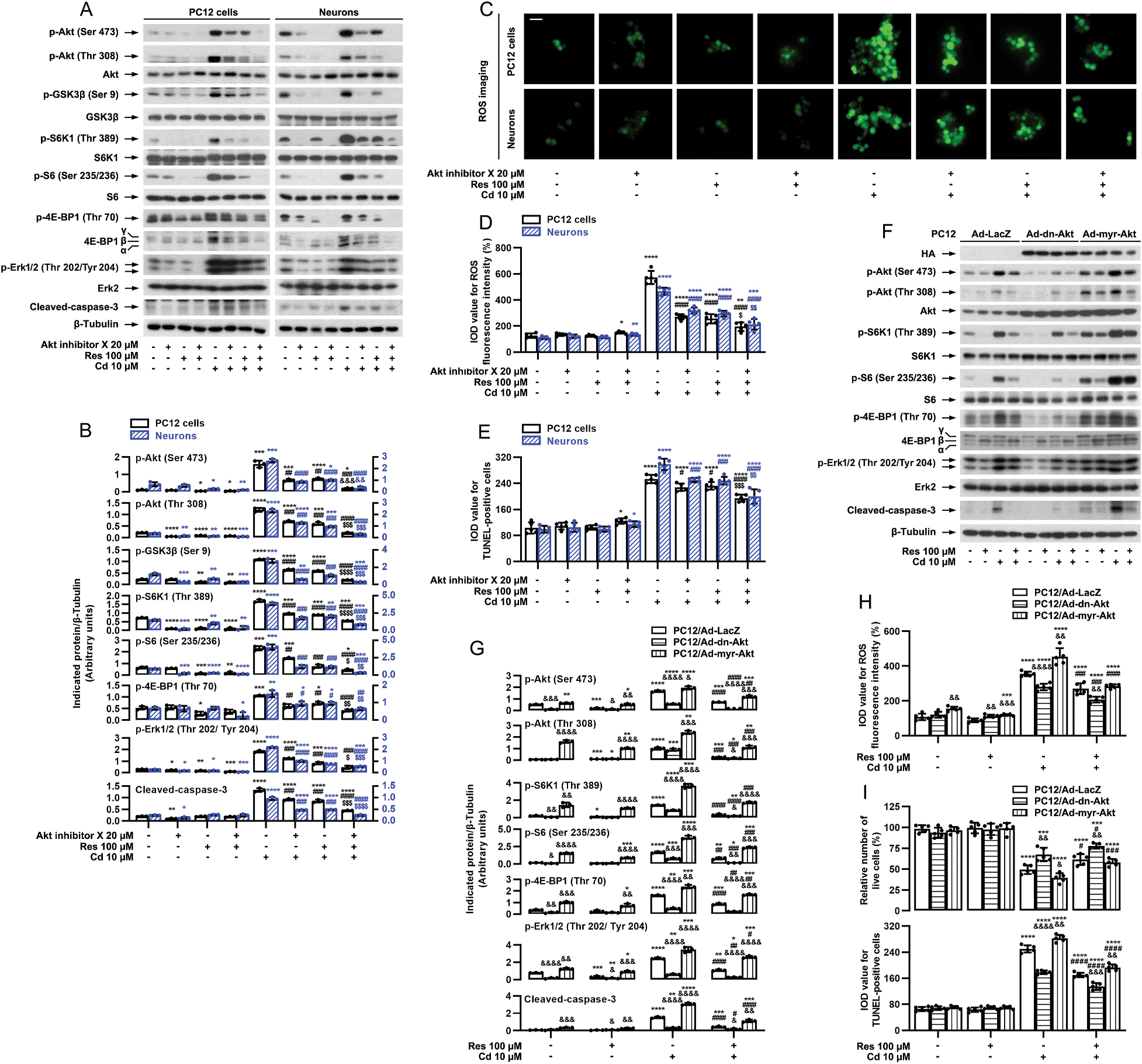
mTORC2-mediated Akt pathway contributes to resveratrol’s inhibition of Cd-induced Erk1/2 activation, ROS generation and apoptosis in neuronal cells. PC12 cells and primary neurons, or PC12 cells infected with Ad-dn-Akt, Ad-myr-Akt or Ad-LacZ (as control), respectively, were pretreated with/without Akt inhibitor X (20 μM) for 1 h and then resveratrol (Res, 100 μM) for 1 h, or pretreated with/without Res for 1 h, followed by exposure to Cd (10 μM) for 4 h (for Western blotting) or 24 h (for ROS imaging, live cell assay and TUNEL staining). A and F) Total cell lysates were subjected to Western blot analysis using indicated antibodies. The blots were probed for β-tubulin as a loading control. Similar results were observed in at least five independent experiments. B and G) The relative densities for p-Akt (Ser473), p-Akt (Thr308), p-GSK3β (Ser9), p-S6K1 (Thr389), p-S6 (Ser235/236), p-4E-BP1 (Thr70), p-Erk1/2 (Thr202/Tyr204), cleaved-caspase-3 to β-tubulin were semi-quantified using NIH image J. C, D and H) Cell ROS was imaged and quantified using an oxidant-sensitive probe CM-H2DCFDA. Scale bar: 20 μm. E and I) Apoptotic cells were evaluated by in situ detection of fragmented DNA using TUNEL staining, and live cells were detected by counting viable cells using trypan blue exclusion. Results are presented as mean ± SEM, n = 3–5. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, difference vs control group; #p < 0.05, ##p < 0.01, ###p < 0.001, ####p < 0.0001, difference vs 10 μM Cd group; $p < 0.05, $$p < 0.01, $$$p < 0.001, $$$$p < 0.0001, difference vs Cd/Akt inhibitor X group or Cd/Res group. &p < 0.05, &&p < 0.01, &&&p < 0.001, &&&&p < 0.0001, Ad-dn-Akt group or Ad-myr-Akt vs Ad-LacZ group.
To further confirm the functional significance of mTORC2-mediated Akt pathway in resveratrol’s resistance to Cd-induced neuronal apoptosis, PC12 cells, infected with recombinant adenoviruses encoding HA-tagged dominant negative Akt (Ad-dn-Akt), constitutively active Akt (Ad-myr-Akt) or LacZ (Ad-LacZ) (as control), were pretreated with/without resveratrol (100 μM) for 1 h, followed by exposure to Cd (10 μM) for 4 h or 24 h. Western blot analysis showed that the infection with Ad-dn-Akt and Ad-myr-Akt, but not Ad-LacZ, led to high expression of HA-tagged Akt mutants (Fig. 9F). The basal phosphorylation level of Akt was significantly decreased and increased by the infection with Ad-dn-Akt and Ad-myr-Akt, respectively, compared to the control infection with Ad-LacZ (Fig. 9F and G). Importantly, ectopic expression of dn-Akt apparently alleviated the basal and/or Cd-induced p-S6K1 [two-way ANOVA: p = 0.0014, F(1.008, 2.017) = 680.7], p-S6 [p = 0.0008, F(1.007, 2.015) = 1192], p-4E-BP1 [p = 0.0025, F(1.096, 2.193) = 267.5], p-Erk1/2 (Thr202/Tyr204) [p = 0.0005, F(1.269, 10.15) = 22.71], cleaved-caspase-3 [p = 0.0048, F(1.008, 2.016) = 200.2], ROS production [p < 0.0151, F(1.554, 21.76) = 142.5], live cell reduction [p < 0.0151, F(1.212, 4.848) = 12.82] and apoptosis [p < 0.0001, F(1.374, 5.497) = 218.0] in the cells, and potentiated the inhibitory activity of resveratrol (Fig. 9F–I), whereas overexpression of myr-Akt had opposite effects, and conferred high resistance to the inhibitory effect of resveratrol (Fig. 9F–I), Taken together, these data indicate that resveratrol inhibits Cd-induced Erk1/2 activation, ROS generation and apoptosis also in part by impeding mTORC2-mediated Akt pathway in neuronal cells.
4. Discussion
Human and animal-based studies have shown that exposure to Cd is able to cause serious health issues, as a possible etiological factor in cancer, cardiovascular, respiratory, renal and neurodegenerative diseases (Chandravanshi et al., 2021; Komatsu et al., 2011; Kwakye et al., 2019; Oggiano et al., 2021; Okuda et al., 1997; Tian et al., 2021; Unsal et al., 2020; Zhang et al., 2021a). For example, Cd crosses the placental barrier and is easy to contact the fetus; moderate or high levels of exposure to this metal during pregnancy may have severe health consequences (Chandravanshi et al., 2021). Cd induces cerebral hemorrhage, increases the permeability of blood-brain barrier, and promotes the formation of abnormal blood vessels in zebrafish (Zhang et al., 2021a); when parental zebrafish is exposed to Cd, the levels of neurotransmitters such as dopamine, serotonin and acetylcholine can be destroyed in zebrafish F1 generation, resulting in developmental neurotoxicity (Tian et al., 2021), implying that Cd-poisoning may severely affect the function of the offspring nervous system. In addition, many data have documented that Cd oxidative stress-related toxicity seems to be a clear cause of damage to many organs (i.e. brain) and tissues in human and other mammals (Baldini et al., 2000; Mouro et al., 2021; Oldereid et al., 1993; Park et al., 2021). Therefore, identifying effective drugs will have a significant impact on prevention and treatment of neurodegenerative diseases caused by Cd exposure.
Resveratrol, a nature-derived compound, possesses numerous bioactivities such as anti-inflammatory, anti-oxidative and antiaging actions, in a variety of species and cell lines (Bastianetto et al., 2015; Guo et al., 2018; Li et al., 2012; Oliveira et al., 2017; Zhou et al., 2021). Studies have shown that single intravenous infusion of resveratrol increases cerebral blood by scavenging free radicals and releasing NO, and protect neurons from cerebral ischemia (Cheng et al., 2009; Lu et al., 2006; Zhang et al., 2008). Resveratrol exerts a neuroprotective action by scavenging ROS in PD model of mice caused by MPTP (Lu et al., 2008). Resveratrol prevents against Cd-induced oxidative damage in mice (Eybl et al., 2006). The findings point out resveratrol’s anti-oxidative neuroprotection. In addition, resveratrol may prevent Cd from triggering multiple signaling pathways and inducing neurotoxicity in the brain (Shati, 2019a; Shati and Alfaifi, 2019). For example, resveratrol reverses Cd-induced hippocampal apoptosis by inhibiting endoplasmic reticulum (ER) stress and activating sirtuin 1 (SIRT1)/AMPK/Akt in rats (Shati, 2019). Resveratrol blocks Tau phosphorylation in rat’s brain by activating PP2A protein and AMPK/PI3K/Akt-induced inhibition of glycogen synthase kinase 3β (GSK3β) (Shati and Alfaifi, 2019). Our group has demonstrated that Cd activates MAPKs and mTORC1/2 signaling network contributing to neuronal cell death (Chen et al., 2008a, 2008b, 2011a; Xu et al., 2015; Zhang et al., 2019), and revealed that resveratrol antagonizes Cd-induced neuronal cell death via PP2A/PP5-inhibited Erk1/2 and JNK pathways (Liu et al., 2015). The data clearly indicate that resveratrol possesses neuroprotective effects in the model of Cd exposure in vitro and in vivo. In the current study, we found that pretreatment with 100 μM of resveratrol for 1 h was able to dramatically detoxicate Cd-triggered phosphorylation of S6K1 (Thr389), S6 (Ser235/236), 4E-BP1 (Thr70) and Akt (Ser473 and Thr308), as well as cleavages of caspase-3, activation of caspases 3/7, the number of TUNEL-positive cells with fragmented DNA and the ratios of apoptotic cells in PC12 cells and primary neurons (Fig. 2), and similar events were also seen in the cells co-treated with resveratrol/Cd simultaneously or pretreated with Cd for 1 h followed by exposure to resveratrol (Fig. 3), suggesting that resveratrol may suppress Cd-induced apoptosis in neuronal cells by blocking mTORC1 and mTORC2 (mTORC1/2) signaling pathways.
mTOR plays a central role in the regulation of cell growth/proliferation, survival and apoptosis (Liu and Sabatini, 2020). A number of studies have described that resveratrol exerts neuroprotection on spinal cord injury (Zhou et al., 2018), stroke (Zhou et al., 2018) and glutamate-induced cortical neuronal cell death (Cho et al., 2014) in rats by inhibiting mTOR pathway. In this study, we confirmed that resveratrol prevented Cd activation of mTOR-dependent neuronal apoptosis, as inhibiting mTORC1 with rapamycin or silencing mTOR potentiated resveratrol’s prevention of Cd-induced phosphorylation of mTOR, S6K1/S6 and 4E-BP1 and apoptosis in neuronal cells (Fig. 4). In addition, pharmacological inhibition of mTORC1/2 with PP242 (mTORC1/2 inhibitor) or disruption of mTORC1 and/or mTORC2 by silencing raptor, rictor or raptor/rictor, respectively, markedly enhanced the resveratrol’s prevention of Cd-induced phosphorylation of Akt, mTOR, S6K1 and/or 4E-BP1, as well as cleaved-caspase-3 and cell death in PC12 cells and/or primary neurons (Figs. 5 and 6). These observations support that resveratrol counteracts Cd neurotoxicity by inhibiting both mTORC1 and mTORC2 pathways.
S6K1 and 4E-BP1 are two best-characterized downstream effector molecules of mTORC1 (Liu and Sabatini, 2020). To verify whether resveratrol attenuates Cd-induced neuronal apoptosis through targeting mTORC1-mediated S6K1 and/or 4E-BP1/eIF4E pathways, the levels or activities of S6K1 and 4E-BP1 were individually manipulated genetically. We demonstrated that downregulation of S6K1 or 4E-BP1, or ectopic expression of constitutively hypophosphorylated 4E-BP1 (4E-BP1–5A) reinforced the resveratrol’s inhibition of Cd-evoked cell death, whereas overexpression of S6K1-ca conferred high resistance to the inhibitory effect of resveratrol (Figs. 7 and 8). The data underscore that both mTORC1-mediated S6K1 and 4E-BP1 pathways are involved in resveratrol’s repression of Cd-induced neuronal apoptosis.
Recent studies have shown that resveratrol achieves neuroprotective effect by inhibiting Akt/GSK3β pathway (Ai et al., 2021; Yan et al., 2021). As Akt is an effector of mTORC2 (Liu and Sabatini, 2020), we also validated whether the Akt pathway may play a role in resveratrol’s inhibition of Cd-triggered neuronal apoptosis. For this, pharmacological inhibition of Akt with Akt inhibitor X or ectopic expression of dn-Akt reinforced resveratrol’s prevention of Cd-induced phosphorylation of GSK3β, S6K1/S6, 4E-BP1, and/or Erk1/2, expression of cleaved-caspase-3, as well as ROS generation, cell viability reduction and/or apoptosis, whereas ectopic expression of myr-Akt rendered resistance to resveratrol’s inhibition of Cd-induced events in PC12 cells and/or primary neurons (Fig. 9). The results confirm that resveratrol inhibits Cd-induced neuronal apoptosis also in part by inhibiting mTORC2-mediated Akt pathway. It is worth mentioning that Akt can activate Erk1/2 (Chetram and Hinton, 2012). Cd-activated Akt contributes to neuronal cell death by inducing ROS generation (Chen et al., 2011a). Resveratrol can competitively combine to free radicals (Liu et al., 2011) and especially, resveratrol can attenuate Cd-elicited lipid peroxidation and ameliorate Cd-impaired antioxidant status in mice (Eybl et al., 2006). Our group has revealed that resveratrol blocks Cd-induced activation of Erk1/2-dependent neuronal cell death (Liu et al., 2015). This study showed that resveratrol repressed Cd-induced Erk1/2 activation, ROS generation and apoptosis also by impeding mTORC2-mediated Akt pathway in neuronal cells (Fig. 9). Collectively, these data imply that resveratrol may prevent Cd-induced neuronal apoptosis at multiple levels, impacting the crosstalk between the ROS, Akt and Erk1/2 pathways. It would be important to determine whether resveratrol’s prevention of Cd-induced ROS generation is caused by inhibition of Akt and/or Akt-Erk1/2 pathways or whether resveratrol’s inhibition of Akt and/or Akt-Erk1/2 pathways results from its directly scavenging ROS, or whether resveratrol affects the interactions between the ROS, Akt and Erk1/2 pathways, thereby suppressing Cd-induced neuronal apoptosis. Additionally, since mTORC2 not only mediates the phosphorylation of Akt, but also regulates the phosphorylation or activity of SGK1, PKCα, small GTPases, and focal adhesion proteins (Cornu et al., 2013; Liu and Sabatini, 2020), more research is also required to define whether any of other mTORC2-mediated signaling molecules is involved in neuroprotection of resveratrol against Cd-poisoning.
Of note, in the present study, we observed that inhibition of mTORC1/2 by PP242 strengthened resveratrol’s protection against Cd-induced apoptosis in neuronal cells. Besides, disruption of both mTORC1 and mTORC2 by silencing raptor and rictor respectively also potentiated resveratrol’s protective effect. It has been reported that resveratrol activates neuronal autophagy by activating AMPK in the ischemic brain (Pineda-Ramirez et al., 2020). Resveratrol alleviates early brain injury after subarachnoid hemorrhage via activating the AMPK/SIRT1/autophagy signaling pathway (Li and Han, 2018). Resveratrol-primed exosomes promote the recovery of motor function in spinal cord injury rats by activating autophagy and inhibiting apoptosis via the PI3K signaling pathway (Fan et al., 2020). The findings suggest that in addition of mTORC1 and mTORC2, resveratrol may inhibit Cd-induced neuronal apoptosis via multiple signaling pathways. This is also supported by our previous observation that resveratrol prevents Cd-induced neuronal cell death by activating PP2A and PP5, leading to suppression of Erk1/2 and JNK pathways (Liu et al., 2015). In the current study, we extended our study using U0126 (5 μM, inhibitor of MKK1/2, upstream kinases of Erk1/2) or SP600125 (20 μM, JNK inhibitor), further demonstrating that there existed more potent protection against Cd-induced phosphorylation of Erk1/2, p-JNK and/or c-Jun, cleavages of caspase-3 and the number of TUNEL-positive cells in PC12 cells and primary neurons co-treated with resveratrol/PP242/U0126 or resveratrol/PP242/SP600125 (Fig. 5). Overall, our results again support that resveratrol inhibits Cd-induced neuronal apoptosis via targeting multiple signaling pathways. Undoubtedly, inhibition of the mTORC1/2 pathways is one of the mechanisms by which resveratrol attenuates Cd-induced neuronal apoptosis. Definitely, more studies are needed to address clinical significance of resveratrol for prevention of Cd neurotoxicity in animal models.
In conclusion, we have shown that neuroprotection of resveratrol against Cd-poisoning acts through dual inhibition of mTORC1/2 signaling pathway. Resveratrol inhibits both mTORC1-mediated S6K1/4E-BP1 and mTORC2-mediated Akt pathways, thereby ameliorating Cd-induced neuronal cell death. Our findings highlight that resveratrol can be exploited for the prevention of Cd toxicity related to neurodegenerative diseases.
Supplementary Material
Acknowledgements
This work was supported in part by the grants from National Natural Science Foundation of China (81873781, 30971486), National Institutes of Health (CA115414), Project for the Priority Academic Program Development of Jiangsu Higher Education Institutions of China (PAPD-14KJB180010), and American Cancer Society (RSG-08–135-01-CNE).
Abbreviations:
- 4E-BP1
eukaryotic initiation factor 4E binding protein 1
- AD
Alzheimer disease
- Akt
protein kinase B (PKB)
- ALS
amyotrophic lateral sclerosis
- AMPK
AMP-activated protein kinase
- Cd
cadmium
- CM-H2DCFDA
5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate
- DMEM
Dulbecco’s Modified Eagle’s Medium
- Erk1/2
extracellular signal-regulated kinases 1/2
- FBS
fetal bovine serum
- GSK3β
glycogen synthase kinase 3β
- HD
Huntington’s disease
- JNK
c-Jun N-terminal kinase
- MAPKs
mitogen-activated protein kinases
- mTOR
mammalian target of rapamycin
- mTORC1/2
mTOR complex 1/2
- PBS
phosphate buffered saline
- PD
Parkinson disease
- PDL
poly-d-lysine
- PI3K
phosphatidylinositol-3-hydroxykinase
- PKCα
protein kinase Cα
- PP2A
protein phosphatase 2A
- PP5
protein phosphatase 5
- Raptor
regulatory-associated protein of mTOR
- Rictor
rapamycin insensitive companion of mTOR
- ROS
reactive oxygen species
- S6K1
ribosomal p70 S6 kinase 1
- SGK1
serum and glucocorticoid-induced kinase 1
- SIRT1
sirtuin 1
- TUNEL
the terminal deoxynucleotidyl transferase (TdT)-mediated deoxyuridine triphosphate (dUTP) nick-end labeling
Footnotes
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
CRediT authorship contribution statement
Chunxiao Liu: Conceptualization, Data curation, Investigation, Methodology, Formal analysis, Project administration, Investigation, Writing – original draft. Ruijie Zhang: Data curation, Investigation, Methodology, Validation, Formal analysis. Liu Yang: Data curation, Investigation, Methodology, Formal analysis. Tong Ji: Data curation, Methodology, Software. Cuilan Zhu: Data curation, Methodology, Resources, Software. Beibei Liu: Methodology, Resources, Software. Hai Zhang: Methodology, Resources, Software. Chong Xu: Methodology, Resources, Software. Nana Zhang: Methodology, Resources. Shile Huang: Conceptualization, Funding acquisition, Supervision, Writing – review & editing. Long Chen: Conceptualization, Formal analysis, Funding acquisition, Supervision, Writing – original draft, Writing – review & editing.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.neuropharm.2022.109236.
Data availability
Data will be made available on request.
References
- Abdullah A, Mohd Murshid N, Makpol S, 2020. Antioxidant modulation of mTOR and sirtuin pathways in age-related neurodegenerative diseases. Mol. Neurobiol. 57 (12), 5193–5207. [DOI] [PubMed] [Google Scholar]
- Ai J, Wang H, Chu P, Shopit A, Niu M, Ahmad N, Tesfaldet T, Wang FH, Fang JN, Li X, Tang SJ, Qing Ju H, Han G, Peng J, Tang Z, 2021. The neuroprotective effects of phosphocreatine on Amyloid β 25–35-induced differentiated neuronal cell death through inhibition of AKT/GSK-3β/Tau/APP/CDK5 pathways in vivo and vitro. Free Radic. Biol. Med. 162, 181–190. [DOI] [PubMed] [Google Scholar]
- Baldini M, Stacchini P, Cubadda F, Miniero R, Parodi P, Facelli P, 2000. Cadmium in organs and tissues of horses slaughtered in Italy. Food Addit. Contam. 17 (8), 679–687. [DOI] [PubMed] [Google Scholar]
- Bar-Sela S, Reingold S, Richter ED, 2001. Amyotrophic lateral sclerosis in a battery-factory worker exposed to cadmium. Int. J. Occup. Environ. Health 7 (2), 109–112. [DOI] [PubMed] [Google Scholar]
- Bastianetto S, Menard C, Quirion R, 2015. Neuroprotective action of resveratrol. Biochim. Biophys. Acta 1852 (6), 1195–1201. [DOI] [PubMed] [Google Scholar]
- Bonithon-Kopp C, Huel G, Moreau T, Wendling R, 1986. Prenatal exposure to lead and cadmium and psychomotor development of the child at 6 years. Neurobehav. Toxicol. Teratol. 8, 307–310. [PubMed] [Google Scholar]
- Chandravanshi L, Shiv K, Kumar S, 2021. Developmental toxicity of cadmium in infants and children: a review. Environ. Anal. Health Toxicol. 36 (1) e2021003–2021000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Liu L, Huang S, 2008a. Cadmium activates the mitogen-activated protein kinase (MAPK) pathway via induction of reactive oxygen species and inhibition of protein phosphatases 2A and 5. Free Radic. Biol. Med. 45 (7), 1035–1044. [DOI] [PubMed] [Google Scholar]
- Chen L, Liu L, Luo Y, Huang S, 2008b. MAPK and mTOR pathways are involved in cadmium-induced neuronal apoptosis. J. Neurochem. 105 (1), 251–261. [DOI] [PubMed] [Google Scholar]
- Chen L, Xu B, Liu L, Luo Y, Yin J, Zhou H, Chen W, Shen T, Han X, Huang S, 2010. Hydrogen peroxide inhibits mTOR signaling by activation of AMPKalpha leading to apoptosis of neuronal cells. Lab. Invest. 90 (5), 762–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Xu B, Liu L, Luo Y, Zhou H, Chen W, Shen T, Han X, Kontos CD, Huang S, 2011a. Cadmium induction of reactive oxygen species activates the mTOR pathway, leading to neuronal cell death. Free Radic. Biol. Med. 50 (5), 624–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Ren Q, Zhang J, Ye Y, Zhang Z, Xu Y, Guo M, Ji H, Xu C, Gu C, Gao W, Huang S, Chen L, 2014. N-acetyl-L-cysteine protects against cadmium-induced neuronal apoptosis by inhibiting ROS-dependent activation of Akt/mTOR pathway in mouse brain. Neuropathol. Appl. Neurobiol. 40 (6), 759–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Xu Y, Xu B, Guo M, Zhang Z, Liu L, Ma H, Chen Z, Luo Y, Huang S, Chen L, 2011b. CaMKII is involved in cadmium activation of MAPK and mTOR pathways leading to neuronal cell death. J. Neurochem. 119 (5), 1108–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng G, Zhang X, Gao D, Jiang X, Dong W, 2009. Resveratrol inhibits MMP-9 expression by up-regulating PPAR alpha expression in an oxygen glucose deprivation-exposed neuron model. Neurosci. Lett. 451 (2), 105–108. [DOI] [PubMed] [Google Scholar]
- Chetram MA, Hinton CV, 2012. PTEN regulation of ERK1/2 signaling in cancer. J. Recept. Signal Transduct. Res. 32 (4), 190–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho KS, Lee EJ, Kwon KJ, Gonzales EL, Kim YB, Cheong JH, Bahn GH, Lee J, Han SH, Kim YT, Shin CY, 2014. Resveratrol down-regulates a glutamate-induced tissue plasminogen activator via Erk and AMPK/mTOR pathways in rat primary cortical neurons. Food Funct. 5 (5), 951–960. [DOI] [PubMed] [Google Scholar]
- Chouit Z, Djellal D, Haddad S, Hanfer M, Hachemi M, Lakroun Z, Chafaa S, Fetoui H, Kebieche M, Soulimani R, 2021. Potentiation of the apoptotic signaling pathway in both the striatum and hippocampus and neurobehavioral impairment in rats exposed chronically to a low-dose of cadmium. Environ. Sci. Pollut. Res. Int. 28 (3), 3307–3317. [DOI] [PubMed] [Google Scholar]
- Ciesielski T, Weuve J, Bellinger DC, Schwartz J, Lanphear B, Wright RO, 2012. Cadmium exposure and neurodevelopmental outcomes in U.S. children. Environ. Health Perspect. 120 (5), 758–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornu M, Albert V, Hall MN, 2013. mTOR in aging, metabolism, and cancer. Curr. Opin. Genet. Dev. 23 (1), 53–62. [DOI] [PubMed] [Google Scholar]
- de la Torre JC, 2021. Deciphering Alzheimer’s disease pathogenic pathway: role of chronic brain hypoperfusion on p-Tau and mTOR. J. Alzheimers Dis. 79 (4), 1381–1396. [DOI] [PubMed] [Google Scholar]
- Eybl V, Kotyzova D, Koutensky J, 2006. Comparative study of natural antioxidants - curcumin, resveratrol and melatonin - in cadmium-induced oxidative damage in mice. Toxicology 225 (2–3), 150–156. [DOI] [PubMed] [Google Scholar]
- Fan Y, Li Y, Huang S, Xu H, Li H, Liu B, 2020. Resveratrol-primed exosomes strongly promote the recovery of motor function in SCI rats by activating autophagy and inhibiting apoptosis via the PI3K signaling pathway. Neurosci. Lett. 736, 135262. [DOI] [PubMed] [Google Scholar]
- Guo D, Xie J, Zhao J, Huang T, Guo X, Song J, 2018. Resveratrol protects early brain injury after subarachnoid hemorrhage by activating autophagy and inhibiting apoptosis mediated by the Akt/mTOR pathway. Neuroreport 29 (5), 368–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedlund B, Gamarra M, Bartfai T, 1979. Inhibition of striatal muscarinic receptors in vivo by cadmium. Brain Res. 168 (1), 216–218. [DOI] [PubMed] [Google Scholar]
- Jiang D, Peng Y, 2021. The protective effect of decoction of Rehmanniae via PI3K/Akt/mTOR pathway in MPP+-induced Parkinson’s disease model cells. J. Recept. Signal Transduct. Res. 41 (1), 74–84. [DOI] [PubMed] [Google Scholar]
- Komatsu F, Kagawa Y, Kawabata T, Kaneko Y, Chimedregzen U, Purvee B, Otgon J, 2011. A high accumulation of hair minerals in Mongolian people: 2(nd) report; influence of manganese, iron, lead, cadmium and aluminum to oxidative stress, Parkinsonism and arthritis. Curr. Aging Sci. 4 (1), 42–56. [DOI] [PubMed] [Google Scholar]
- Kwakye GF, Jimenez JA, Thomas MG, Kingsley BA, Mc IM, Saito MA, Korley EM, 2019. Heterozygous huntingtin promotes cadmium neurotoxicity and neurodegeneration in striatal cells via altered metal transport and protein kinase C delta dependent oxidative stress and apoptosis signaling mechanisms. Neurotoxicology 70, 48–61. [DOI] [PubMed] [Google Scholar]
- Li F, Gong Q, Dong H, Shi J, 2012. Resveratrol, a neuroprotective supplement for Alzheimer’s disease. Curr. Pharmaceut. Des. 18 (1), 27–33. [DOI] [PubMed] [Google Scholar]
- Li Z, Han X, 2018. Resveratrol alleviates early brain injury following subarachnoid hemorrhage: possible involvement of the AMPK/SIRT1/autophagy signaling pathway. Biol. Chem. 399 (11), 1339–1350. [DOI] [PubMed] [Google Scholar]
- Liu C, Shi Z, Fan L, Zhang C, Wang K, Wang B, 2011. Resveratrol improves neuron protection and functional recovery in rat model of spinal cord injury. Brain Res. 1374, 100–109. [DOI] [PubMed] [Google Scholar]
- Liu C, Zhang R, Sun C, Zhang H, Xu C, Liu W, Gao W, Huang S, Chen L, 2015. Resveratrol prevents cadmium activation of Erk1/2 and JNK pathways from neuronal cell death via protein phosphatases 2A and 5. J. Neurochem. 135 (3), 466–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu GY, Sabatini DM, 2020. mTOR at the nexus of nutrition, growth, ageing and disease. Nat. Rev. Mol. Cell Biol. 21 (4), 183–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Chen L, Chung J, Huang S, 2008. Rapamycin inhibits F-actin reorganization and phosphorylation of focal adhesion proteins. Oncogene 27 (37), 4998–5010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Li F, Cardelli JA, Martin KA, Blenis J, Huang S, 2006. Rapamycin inhibits cell motility by suppression of mTOR-mediated S6K1 and 4E-BP1 pathways. Oncogene 25 (53), 7029–7040. [DOI] [PubMed] [Google Scholar]
- Liu M, Wilk SA, Wang A, Zhou L, Wang RH, Ogawa W, Deng C, Dong LQ, Liu F, 2010. Resveratrol inhibits mTOR signaling by promoting the interaction between mTOR and DEPTOR. J. Biol. Chem. 285 (47), 36387–36394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu KT, Chiou RY, Chen LG, Chen MH, Tseng WT, Hsieh HT, Yang YL, 2006. Neuroprotective effects of resveratrol on cerebral ischemia-induced neuron loss mediated by free radical scavenging and cerebral blood flow elevation. J. Agric. Food Chem. 54 (8), 3126–3131. [DOI] [PubMed] [Google Scholar]
- Lu KT, Ko MC, Chen BY, Huang JC, Hsieh CW, Lee MC, Chiou RY, Wung BS, Peng CH, Yang YL, 2008. Neuroprotective effects of resveratrol on MPTP-induced neuron loss mediated by free radical scavenging. J. Agric. Food Chem. 56 (16), 6910–6913. [DOI] [PubMed] [Google Scholar]
- Mostafa DG, Khaleel EF, Badi RM, Abdel-Aleem GA, Abdeen HM, 2019. Rutin hydrate inhibits apoptosis in the brains of cadmium chloride-treated rats via preserving the mitochondrial integrity and inhibiting endoplasmic reticulum stress. Neurol. Res. 41 (7), 594–608. [DOI] [PubMed] [Google Scholar]
- Mothe-Satney I, Yang D, Fadden P, Haystead TA, Lawrence JC Jr., 2000. Multiple mechanisms control phosphorylation of PHAS-I in five (S/T)P sites that govern translational repression. Mol. Cell Biol. 20 (10), 3558–3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouro VGS, Ladeira LCM, Lozi AA, de Medeiros TS, Silva MR, de Oliveira EL, de Melo F, da Matta SLP, 2021. Different routes of administration lead to different oxidative damage and tissue disorganization levels on the subacute cadmium toxicity in the liver. Biol. Trace Elem. Res. 199 (12), 4624–4634. [DOI] [PubMed] [Google Scholar]
- Oggiano R, Pisano A, Sabalic A, Farace C, Fenu G, Lintas S, Forte G, Bocca B, Madeddu R, 2021. An overview on amyotrophic lateral sclerosis and cadmium. Neurol. Sci. 42 (2), 531–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda B, Iwamoto Y, Tachibana H, Sugita M, 1997. Parkinsonism after acute cadmium poisoning. Clin. Neurol. Neurosurg. 99 (4), 263–265. [DOI] [PubMed] [Google Scholar]
- Oldereid NB, Thomassen Y, Attramadal A, Olaisen B, Purvis K, 1993. Concentrations of lead, cadmium and zinc in the tissues of reproductive organs of men. J. Reprod. Fertil. 99 (2), 421–425. [DOI] [PubMed] [Google Scholar]
- Oliveira ALB, Monteiro VVS, Navegantes-Lima KC, Reis JF, Gomes RS, Rodrigues DVS, Gaspar SLF, Monteiro MC, 2017. Resveratrol role in autoimmune disease-A mini-review. Nutrients 9 (12), 1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, Lee BM, Kim HS, 2021. Potential protective roles of curcumin against cadmium-induced toxicity and oxidative stress. J. Toxicol. Environ. Health B Crit. Rev. 24 (3), 95–118. [DOI] [PubMed] [Google Scholar]
- Pineda-Ramirez N, Alquisiras-Burgos I, Ortiz-Plata A, Ruiz-Tachiquín ME, Espinoza-Rojo M, Aguilera P, 2020. Resveratrol activates neuronal autophagy through AMPK in the ischemic brain. Mol. Neurobiol. 57 (2), 1055–1069. [DOI] [PubMed] [Google Scholar]
- Pinheiro Junior JEG, Moraes PZ, Rodriguez MD, Simoes MR, Cibin F, Pinton S, Barbosa Junior F, Pecanha FM, Vassallo DV, Miguel M, Wiggers GA, 2020. Cadmium exposure activates NADPH oxidase, renin-angiotensin system and cyclooxygenase 2 pathways in arteries, inducing hypertension and vascular damage. Toxicol. Lett. 333, 80–89. [DOI] [PubMed] [Google Scholar]
- Poliandri AH, Esquifino AI, Cano P, Jimenez V, Lafuente A, Cardinali DP, Duvilanski BH, 2006. In vivo protective effect of melatonin on cadmium-induced changes in redox balance and gene expression in rat hypothalamus and anterior pituitary. J. Pineal Res. 41 (3), 238–246. [DOI] [PubMed] [Google Scholar]
- Puissant A, Robert G, Fenouille N, Luciano F, Cassuto JP, Raynaud S, Auberger P, 2010. Resveratrol promotes autophagic cell death in chronic myelogenous leukemia cells via JNK-mediated p62/SQSTM1 expression and AMPK activation. Cancer Res. 70 (3), 1042–1052. [DOI] [PubMed] [Google Scholar]
- Sarroca S, Gatius A, Rodriguez-Farre E, Vilchez D, Pallas M, Grinan-Ferre C, Sanfeliu C, Corpas R, 2021. Resveratrol confers neuroprotection against high-fat diet in a mouse model of Alzheimer’s disease via modulation of proteolytic mechanisms. J. Nutr. Biochem. 89, 108569. [DOI] [PubMed] [Google Scholar]
- Schubert CR, Pinto AA, Paulsen AJ, Cruickshanks KJ, 2021. Exposure to cadmium, lead, and tobacco smoke and the 10-year cumulative incidence of olfactory impairment: the beaver dam offspring study. JAMA Otolaryngol. Head Neck Surg. 147 (6), 510–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang C, Zhou H, Liu W, Shen T, Luo Y, Huang S, 2020. Iron chelation inhibits mTORC1 signaling involving activation of AMPK and REDD1/Bnip3 pathways. Oncogene 39 (29), 5201–5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shati AA, 2019. Resveratrol protects against cadmium chloride-induced hippocampal neurotoxicity by inhibiting ER stress and GAAD 153 and activating sirtuin 1/AMPK/Akt. Environ. Toxicol. 34 (12), 1340–1353. [DOI] [PubMed] [Google Scholar]
- Shati AA, Alfaifi MY, 2019. Trans-resveratrol inhibits Tau phosphorylation in the brains of control and cadmium chloride-treated rats by activating PP2A and PI3K/Akt induced-inhibition of GSK3beta. Neurochem. Res. 44 (2), 357–373. [DOI] [PubMed] [Google Scholar]
- Smedman M, Potempska A, Rubenstein R, Ju W, Ramakrishna N, Denman RB, 1997. Effects of cadmium, copper, and zinc and beta APP processing and turnover in COS-7 and PC12 cells. Relationship to Alzheimer disease pathology. Mol. Chem. Neuropathol. 31 (1), 13–28. [DOI] [PubMed] [Google Scholar]
- Su CF, Jiang L, Zhang XW, Iyaswamy A, Li M, 2021. Resveratrol in rodent models of Parkinson’s disease: a systematic review of experimental studies. Front. Pharmacol. 12, 644219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian J, Hu J, Liu D, Yin J, Chen M, Zhou L, Yin H, 2021. Cadmium chloride-induced transgenerational neurotoxicity in zebrafish development. Environ. Toxicol. Pharmacol. 81, 103545. [DOI] [PubMed] [Google Scholar]
- Tsuchiya K, Seki Y, Sugita M, 1976. Cadmium concentrations in the organs and tissues of cadavers from accidental deaths. Keio J. Med. 25 (2), 83–90. [DOI] [PubMed] [Google Scholar]
- Unsal V, Dalkiran T, Cicek M, Kolukcu E, 2020. The role of natural antioxidants against reactive oxygen species produced by cadmium toxicity: a review. Adv. Pharmaceut. Bull. 10 (2), 184–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Liu C, Liu L, Zhang R, Zhang H, Chen S, Luo Y, Chen L, Huang S, 2015. Rapamycin prevents cadmium-induced neuronal cell death via targeting both mTORC1 and mTORC2 pathways. Neuropharmacology 97, 35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Wang X, Zhu Y, Dong X, Liu C, Zhang H, Liu L, Huang S, Chen L, 2016. Rapamycin ameliorates cadmium-induced activation of MAPK pathway and neuronal apoptosis by preventing mitochondrial ROS inactivation of PP2A. Neuropharmacology 105, 270–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Liu C, Chen S, Ye Y, Guo M, Ren Q, Liu L, Zhang H, Xu C, Zhou Q, Huang S, Chen L, 2014. Activation of AMPK and inactivation of Akt result in suppression of mTOR-mediated S6K1 and 4E-BP1 pathways leading to neuronal cell death in in vitro models of Parkinson’s disease. Cell. Signal. 26 (8), 1680–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C, Yu H, Liu Y, Wu P, Wang C, Zhao H, Yang K, Shao Q, Zhong Y, Zhao W, Li J, Liu N, Di J, Li C, Bao L, Gao C, 2021. c-Abl tyrosine kinase-mediated neuronal apoptosis in subarachnoid hemorrhage by modulating the LRP-1-dependent Akt/GSK3β survival pathway. J. Mol. Neurosci. 71 (12), 2514–2525. [DOI] [PubMed] [Google Scholar]
- Zhang H, Dong X, Zhao R, Zhang R, Xu C, Wang X, Liu C, Hu X, Huang S, Chen L, 2019. Cadmium results in accumulation of autophagosomes-dependent apoptosis through activating Akt-impaired autophagic flux in neuronal cells. Cell. Signal. 55, 26–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Schools GP, Lei T, Wang W, Kimelberg HK, Zhou M, 2008. Resveratrol attenuates early pyramidal neuron excitability impairment and death in acute rat hippocampal slices caused by oxygen-glucose deprivation. Exp. Neurol. 212 (1), 44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Xu Z, Wen L, Lei D, Li S, Wang J, Huang J, Wang N, Durkan C, Liao X, Wang G, 2021a. Cadmium-induced dysfunction of the blood-brain barrier depends on ROS-mediated inhibition of PTPase activity in zebrafish. J. Hazard Mater. 412, 125198. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Sun X, Wang K, Yu Y, Zhang L, Zhang K, Gu J, Yuan X, Song G, 2021b. Hydrogen-saturated saline mediated neuroprotection through autophagy via PI3K/AKT/mTOR pathway in early and medium stages of rotenone-induced Parkinson’s disease rats. Brain Res. Bull. 172, 1–13. [DOI] [PubMed] [Google Scholar]
- Zhou DD, Luo M, Huang SY, Saimaiti A, Shang A, Gan RY, Li HB, 2021. Effects and mechanisms of resveratrol on aging and age-related diseases. Oxid. Med. Cell. Longev. 2021, 9932218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Huo X, Botchway BOA, Xu L, Meng X, Zhang S, Liu X, 2018. Beneficial effects of resveratrol-mediated inhibition of the mTOR pathway in spinal cord injury. Neural Plast. 2018, 7513748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Liu C, Liu W, Zhang H, Zhang R, Liu J, Zhang J, Xu C, Liu L, Huang S, Chen L, 2015. Rotenone induction of hydrogen peroxide inhibits mTOR-mediated S6K1 and 4E-BP1/eIF4E pathways, leading to neuronal apoptosis. Toxicol. Sci. 143 (1), 81–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.


