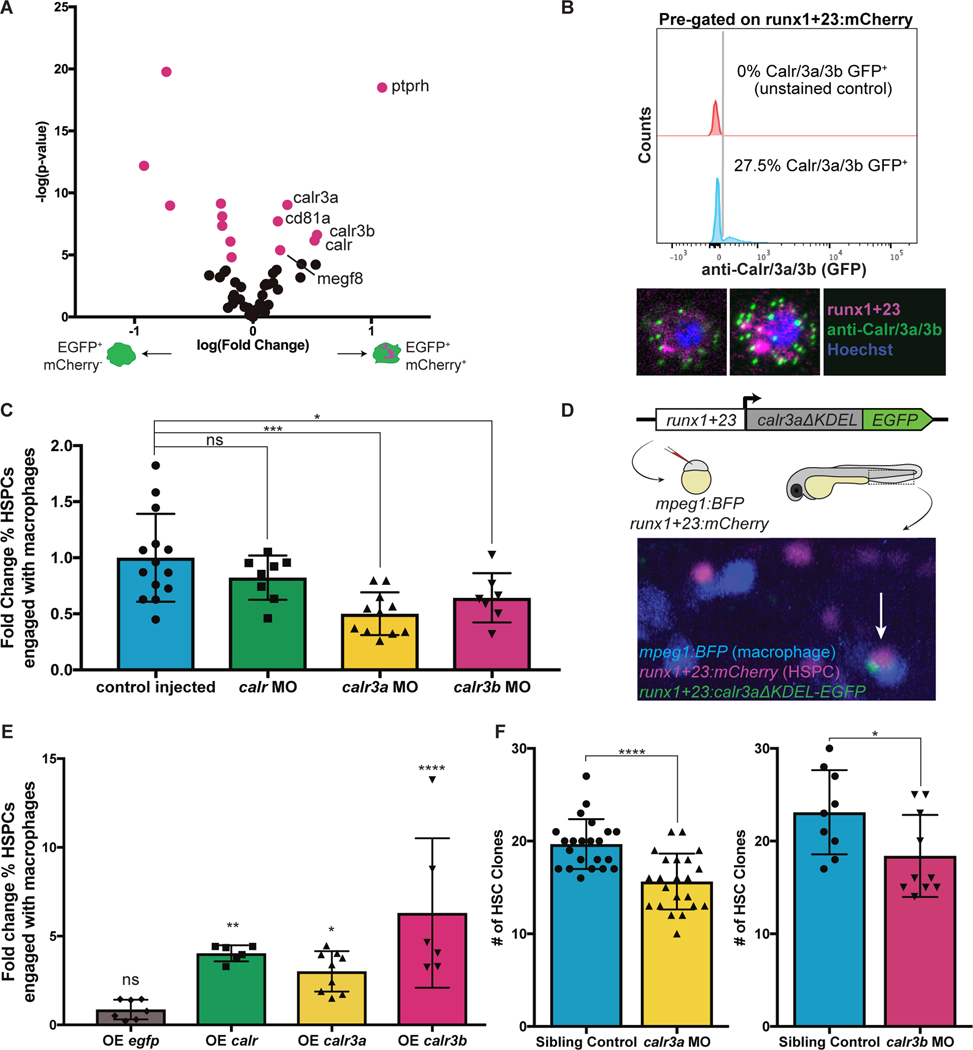
Fig. 3. Calreticulin drives HSPC-macrophage interactions to regulate clonality.
(A) Analysis of differentially enriched potential surface proteins from interacting macrophages identifies three paralogs of Calreticulin. (B) Flow cytometry shows ~30% of runx1+23:mCherry+ HSPCs stain for surface Calreticulin. (C) Morpholino knock-down of calr3a or calr3b significantly reduces the fraction of HSPCs interacting with macrophages at any one time. Mean +/− s.d., One-way ANOVA with Dunnett’s multiple comparisons test; *P<0.05, ***P<0.001. (D) Calreticulin paralogs without the ER-retention KDEL sequence were fused to EGFP, driven by the HSPC-specific runx1+23 enhancer, and injected into stable runx1+23:mCherry;mpeg1:BFP embryos. Mosaic animals overexpress Calreticulin in a random subset of HSPCs. Arrow indicates an HSPC overexpressing calr3a engaged by a macrophage. (E) HSPCs overexpressing calr, calr3a, or calr3b are more frequently engaged by macrophages compared to non-overexpressing HSPCs in the same embryos. Overexpressing egfp alone has no effect. Mean +/− s.d., One-way ANOVA with Dunnett’s multiple comparisons test; *P<0.05, **P<0.01, ****P<0.0001. (F) Knock-down of calr3a or calr3b reduces the number of HSC clones that contribute to adult hematopoiesis. Mean +/− s.d., Unpaired t test; *P<0.05, ****P<0.0001.