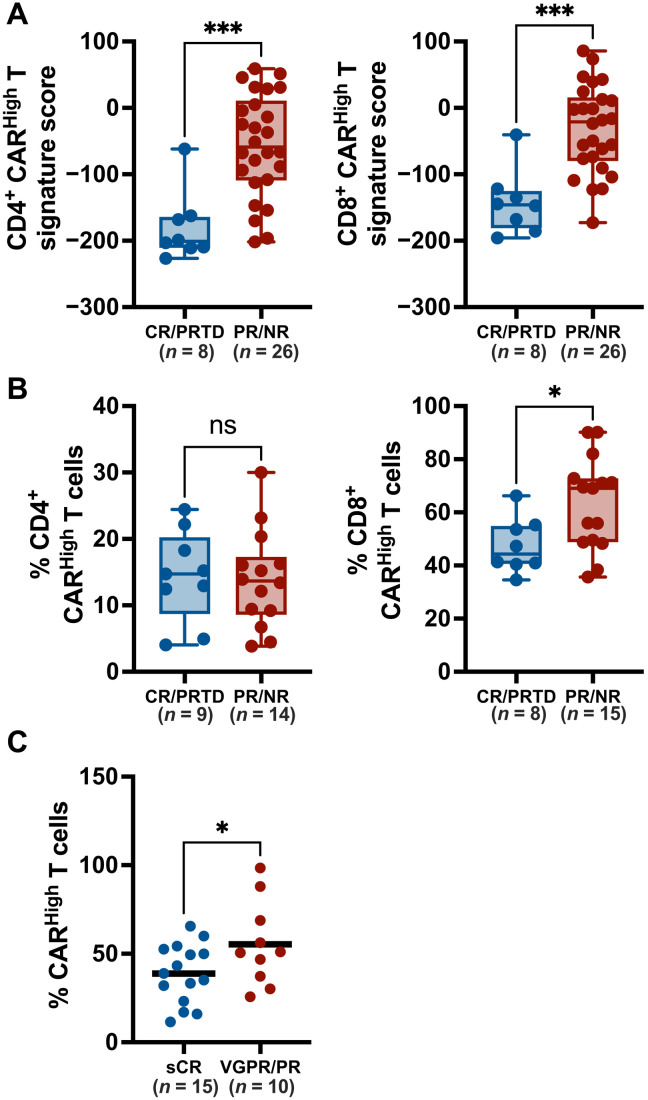
Fig. 7. Increased CAR levels negatively affect clinical response of CAR T therapies.
To evaluate the impact of increased CAR density on the clinical response, we quantify the presence of CARHigh T cells in the infusion products of several clinical trials. (A) The gene signatures developed in this work, associated to CD4+ and CD8+ CARHigh T cells, were applied to 34 infusion products from adult CLL patients treated with CTL019. Signature score for each CAR T cell product is represented for both CD4+ (left) and CD8+ (right) gene signatures, in patients divided according to clinical response into CR/PRTD (complete response/partial response with transformed disease) and PR/NP (partial response/nonresponse). Products from patients with poor clinical response (PR/NR) presented a significant higher score of both CARHigh T signatures. (B) Our gene signature was applied to available scRNA-seq dataset from 24 CAR T infusion products from adult DLBCL patients treated with axi-cel. Percentage of CD4+ (left) and CD8+ (right) CARHigh T cells present for each CAR T cell product is represented in patients divided according to clinical response as described in (A). Products from PR/NR patients were significantly enriched in CD8+ CARHigh T cells. (C) The number of CAR molecules per cell in 25 anti-BCMA CAR T infusion products from CARTBCMA-HCB-01 clinical trial was quantified by FACS. CARHigh T cells were defined as cells with >5000 molecules per cell (see Materials and Methods). Percentage of CARHigh T cells for each infusion product is represented in patients divided into sCR (stringent complete response) and VGPR (very good PR)/PR according to clinical response. Patients with sCR showed a significantly decreased number of CARHigh T cells. Unpaired t tests (A to C). *P < 0.05 and ***P < 0.001.