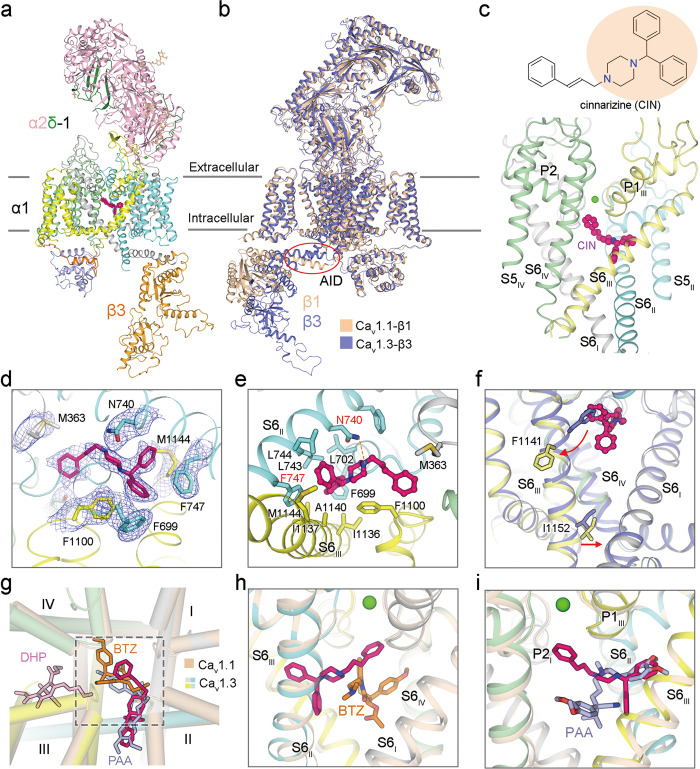
Fig. 1. Pore blockade of human Cav1.3 by cinnarizine.
a Overall structure of Cav1.3 bound to cinnarizine. The complex comprises transmembrane α1 (gray, cyan, yellow, and pale green for Repeats I–IV, respectively), extracellular α2δ-1 (light pink for α2, and green for δ), and cytosolic β3 subunits (bright orange). Cinnarizine and Ca2+ ions are shown as magenta, and green spheres. Wheat sticks indicate N-glycans on the α2δ-1 subunit. b Superimposition of rabbit Cav1.1 complex (colored wheat, PDB: 5GJV) and human Cav1.3 complex (colored purple). Despite treatment with 150 μM cp-PYT before cryo-sample preparation, there is no density for the drug in the EM map of Cav1.3. This structure will thus serve as a reference to study the conformational changes upon cinnarizine binding, as shown in panel f. Red circle highlights the minor structural shift at the AID. c Cinnarizine is accommodated in the central cavity of the PD. Chemical structure of cinnarizine is shown, with the core DPP group shaded light orange. d EM map for cinnarizine and surrounding residues. The densities, shown as blue meshes, are contoured at 4 σ in PyMol. e Molecular details for cinnarizine coordination. A potential hydrogen bond is indicated by orange dashes. f Local conformational changes upon cinnarizine binding. Red arrows indicate the structural shift of S6III upon cinnarizine binding. g The binding mode of cinnarizine is different from that of DHP drugs and other pore blockers. Shown here is an extracellular view of superimposed structures of Cav1.3 (domain-colored) bound to cinnarizine and Cav1.1 (colored wheat) bound to amlodipine (DHP drug, pink sticks, PDB: 7JPX), diltiazem (BTZ, orange sticks, PDB: 6JPB), and verapamil (PAA, light blue sticks, PDB: 6JPA). h Orthogonal binding pockets for cinnarizine and BTZ. i PAA partially overlaps with cinnarizine.