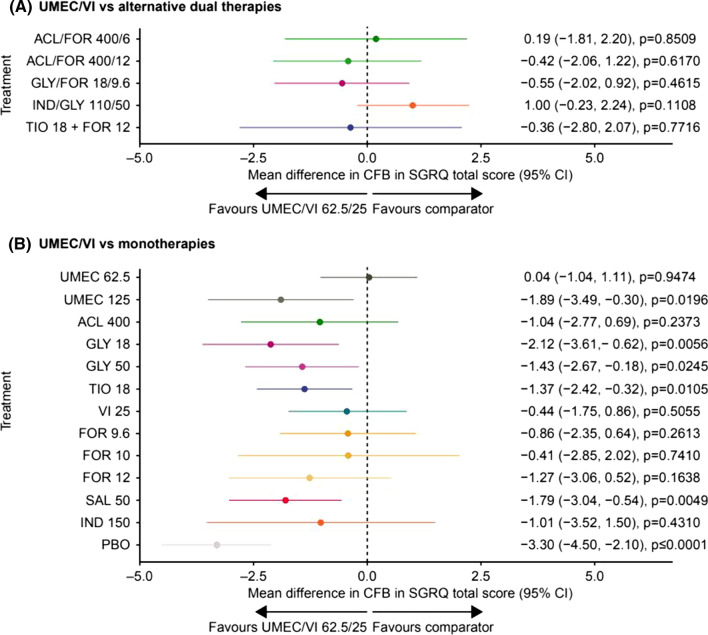
Fig. 3.
Fixed effects model of mean difference in change from baseline in SGRQ total score of UMEC/VI versus a dual therapy and b monotherapy at 24 weeks. Assessment of heterogeneity/inconsistency: I2 = 22.49%; Q = 32.25; p = 0.1508. ACL aclidinium, CFB change from baseline, CI confidence interval, FOR formoterol fumarate, FP fluticasone propionate, GLY glycopyrronium, IND indacaterol, PBO placebo, SAL salmeterol, SGRQ St George’s Respiratory Questionnaire, TIO tiotropium, UMEC umeclidinium, VI vilanterol