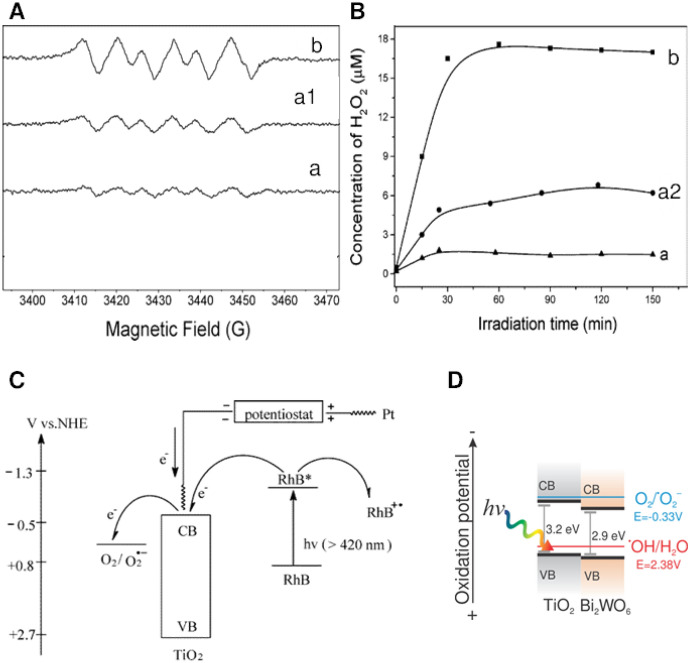
Fig. 4.
ESR spectra for superoxide radical (·O–2/HOO·) adducts with DMPO during photodegradation of Rhodamine B (RhB) (A), the concentration profiles for the formation of H2O2 during the degradation of RhB (10 μM) (B), the proposed mechanism of the degradation of RhB under visible-light irradiation (C), and example VB and CB positions for TiO2 and Bi2WO6 and the potential of the radicals’ redox couples ·OH/H2O and O2/·O2−. In ESR spectra, (a) shows 0.6 V versus saturated calomel electrode (SCE) under visible irradiation; (a1) −0.6 V versus SCE in the dark; (b) −0.6 V versus SCE under visible irradiation. In H2O2 concentration profiles, (a) shows profile of 0.6 V versus SCE under visible irradiation; (a2) with only electrolysis at a bias of −0.6 V versus SCE; (b) under visible light and at a bias of −0.6 V versus SCE [22]
Reproduced with permission from the American Chemical Society