Abstract
Inflammation and oxidative stress play major roles in healthy and pathological pregnancy. Environmental exposure to chemical pollutants may adversely affect maternal and fetal health in pregnancy by dysregulating these critical underlying processes of inflammation and oxidative stress. Oxylipins are bioactive lipids that play a major role in regulating inflammation and increasing lines of evidence point towards an importance in pregnancy. The biosynthetic production of oxylipins requires oxygenation of polyunsaturated fatty acids, which can occur through several well-characterized enzymatic and nonenzymatic pathways. This review describes the state of the science of epidemiologic evidence on oxylipin production in pregnancy and its association with 1) key pregnancy outcomes and 2) environmental exposures. We searched PubMed for studies of pregnancy that measured one or more oxylipin analytes during pregnancy or delivery. We evaluated oxylipin associations with three categories of adverse pregnancy outcomes, including preeclampsia, preterm birth, and fetal growth restriction, along with several categories of environmental pollutants. The majority of studies evaluated one to two oxylipins, most of which focused on oxylipins produced from nonenzymatic processes of oxidative stress. However, an increasing number of recent studies have leveraged technological advancements to profile a large number of oxylipins produced from distinct biosynthetic pathways. Although the literature indicated robust evidence that oxylipins produced via nonenzymatic pathways are associated with pregnancy outcomes and environmental exposures, evidence for enzymatically produced oxylipins showed that associations may differ between biosynthetic pathways. Along with summarizing this evidence, we review promising therapeutic options to regulate oxylipin production and provide a set of recommendations for future epidemiologic studies in these research areas. Further evidence is needed to improve our understanding of how oxylipins may act as key biological mediators for the adverse effects of environmental pollutants on pregnancy outcomes.
Keywords: Inflammation, oxidative stress, oxylipin, eicosanoid, pregnancy, environmental exposure
1. Introduction
Regulation of inflammation and oxidative stress is an essential component of a healthy pregnancy. Natural changes in maternal inflammation during pregnancy help to ensure maintenance of both maternal and fetal health (Mor et al., 2011). However, when inflammation is dysregulated, it presents a pregnancy risk due to an array of downstream physiological consequences, including disruptions in immune function, vascular physiology, endocrine activity, and fetal development (Robinson and Klein, 2012; Ander et al., 2019). Chronic inflammation stemming from unresolved acute inflammatory responses is recognized to be an early determinant of disease risk in pregnancy for outcomes such as preeclampsia, preterm birth, and fetal growth restriction (Mor et al., 2011; Furman et al., 2019). Common clinical biomarkers such as C-reactive protein and tumor necrosis factor alpha provide an indicator of elevated systemic inflammation and have been associated with disease in pregnancy, but provide limited insight into critical upstream inflammatory processes (Furman et al., 2019). Oxylipins are a diverse class of bioactive lipids that play an important upstream role in regulating inflammation and oxidative stress (Dennis and Norris, 2015). Inflammation is physiologically mediated by oxylipins via direct effects on cells and tissues (e.g., vasoconstriction, vasodilation), along with the indirect effects posed on immune function (Esser-von Bieren, 2017). The role of certain oxylipins in normal pregnancy, particularly in the time periods near parturition, has been well characterized (Mosaad et al., 2020). However, increased understanding about the role oxylipins may play in the early origins of pregnancy disorders is needed to understand downstream effects of dysregulated inflammation.
There is also growing epidemiologic evidence that important pregnancy disorders (e.g., preeclampsia, preterm birth, fetal growth restriction) are influenced by exposure to chemical pollutants in the environment (Ferguson and Chin, 2017; Rosen et al., 2018; Kamai et al., 2019). However, the underlying biological mechanisms linking environmental chemical exposures to pregnancy outcomes are still uncertain. Dysregulated inflammation and oxidative stress are primarily driven by non-genetic factors (Brodin et al., 2015). Thus, a leading hypothesis is that inflammation and oxidative stress play a central role in mediating the effects of environmental exposures on pregnancy outcomes (Romero et al., 2007; Hantsoo et al., 2019). Improved understanding of the links between inflammation and adverse pregnancy outcomes, as well as how chemical exposures influence inflammation, may provide critical evidence to comprehensively understand and predict the downstream effects of chemical exposures.
In this state-of-the-art review (Grant and Booth, 2009), we aim to summarize evidence from recent epidemiologic studies investigating links between circulating oxylipins, maternal and fetal outcomes, and chemical exposures in pregnancy. We evaluate the hypothesis that oxylipins act as important biological mediators of the relationship between environmental exposures and pregnancy disorders. We identified studies in PubMed using a combination of search keywords for oxylipins (i.e., oxylipin, eicosanoid, or bioactive lipid, isoprostane), overall health effects (i.e., inflammation or oxidative stress), and related pregnancy outcomes (i.e., preeclampsia, preterm, gestational age, intrauterine growth restriction, or fetal growth). We restricted our search results to studies in human populations and refined results to include only studies that evaluated oxylipins in samples collected during pregnancy or delivery. Given important advances to lipidomic technologies for accurately identifying and quantifying oxylipins have occurred relatively recently (Quehenberger and Dennis, 2011), we restricted our results to studies published since 2010 to evaluate the most up-to-date evidence. First, we review studies that evaluate associations for preeclampsia, preterm birth, and fetal growth outcomes. Second, we summarize evidence from environmental epidemiology studies of pregnancy that evaluate associations with oxylipins. Third, we evaluate potential therapeutic options to modulate oxylipin production in pregnancy. Finally, we provide a set of recommendations for future epidemiologic studies to build upon this knowledge by generating translatable evidence to improve pregnancy outcomes.
2. Biosynthetic pathways of oxylipin production and connection to inflammation and oxidative stress
To contextualize the findings presented in this review, Figure 1 summarizes the major biosynthetic pathways of oxylipin production and the primary effects oxylipins along these pathways have on inflammation or oxidative stress. Oxylipins are a large class of bioactive lipids produced from the oxygenation of polyunsaturated fatty acids (PUFAs), including the primary omega-6 (i.e., linoleic acid [LA], arachidonic acid [AA]) and omega-3 (i.e., docosahexaenoic acid [DHA], and eicosapentaenoic acid [EPA]) PUFAs (Buczynski et al., 2009; Gabbs et al., 2015). The oxygenation of PUFAs to biosynthesize oxylipins can occur nonenzymatically via free radical peroxidation (Milne et al., 2015), or enzymatically by cytochrome P450 (CYP), lipoxygenase (LOX), and cyclooxygenase (COX) enzymes (Buczynski et al., 2009; Gabbs et al., 2015). Recent advances in mass spectrometry-based lipidomic technologies have provided the novel ability to identify and quantify a vast array of distinct plasma oxylipins with high resolution (Quehenberger and Dennis, 2011). The ability to distinguish oxylipins with similar molecular structures has provided a critical step forward in addressing underlying health effects, as seemingly small differences in structure between oxylipins can produce opposing inflammatory effects (Serhan, 2014; Dennis and Norris, 2015). For example, CYP epoxidation of AA produces epoxyeicosatrienoic acids (EETs), which can occur as varying regioisomers (i.e., 5,6-, 8,9-, 11,12-, or 14, 15-EETs) and have anti-inflammatory, angiogenic, and vasodilatory properties (Li et al., 2021). However, CYP hydroxylation of AA can alternatively produce 20-hydroxyeicosatetraenoic acid (HETE) that has important, and contrasting, pro-inflammatory and vasoconstrictive effects (Wu et al., 2014).
Figure 1.
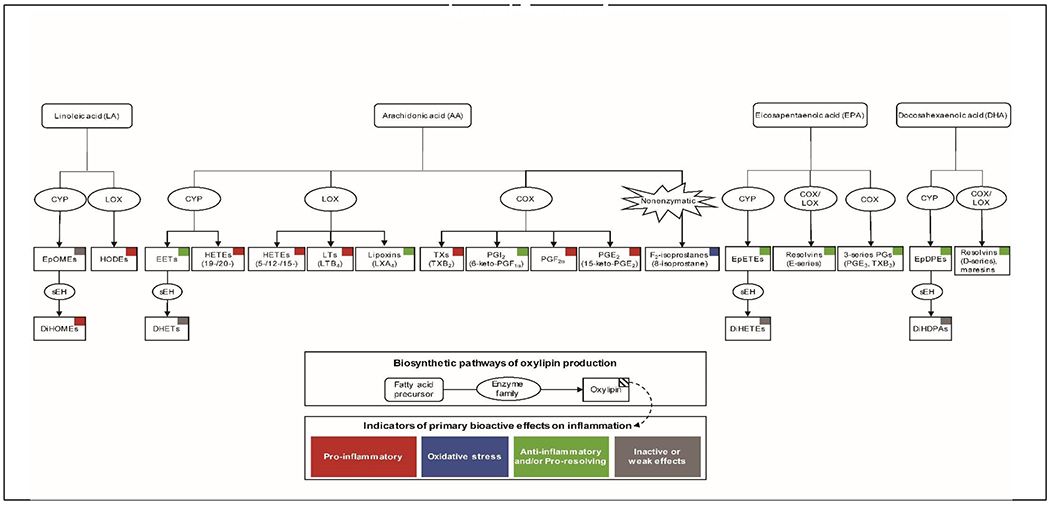
Major biosynthetic pathways of oxylipin production and the bioactive effects of oxylipins on inflammation
Please note that after aspirin-acetylation, COX acts similar to 15-LOX to produce pro-resolving mediators shown (e.g., D-series resolvins, maresins) and not shown (e.g., protectins, neuroprotectins) in the figure.
Abbreviations: 8-isoprostane, 8-iso-prostaglandin-F2α; AA, arachidonic acid; COX, cyclooxygenase; CYP, cytochrome P450; DHA, docosahexaenoic acid; DHET, dihydroxy-eicosatrienoic acid; DiHDPA, dihydroxy-docosapentaenoic acid; DiHETE, dihydroxy-eicosatetraenoic acid; DiHOME, dihydroxy-octadecenoic acid; EET, epoxyeicosatrienoic acid; EPA, eicosapentaenoic acid; EpDPE, epoxy-docosapentaenoic acid; EpETE, epoxy-eicosatetraenoic acid; EpOME, epoxy-octadecenoic acid; HETE, hydroxyeicosatetraenoic acid; HODE, hydroxy-octadecadienoic acid; LA, linoleic acid; LOX, lipoxygenase; LT, leukotriene; LX, lipoxin; PG, prostaglandin; PGI, prostacyclin; TX, thromboxane
For the purposes of this review, we have categorized oxylipins based on enzymatic or nonenzymatic production to reflect processes of inflammation and oxidative stress, respectively. Nonenzymatic production of F2-isoprostanes, particularly 8-iso-prostaglandin-F2α (8-isoprostane), is well-recognized to reflect underlying oxidative stress (Milne et al., 2015). While circulating 8-isoprostane in humans is mostly derived from oxidative stress, it is possible for a very small proportion of 8-isoprostane to be enzymatically derived from COX metabolism (van’t Erve et al., 2015). Thus, our separation of oxylipins by distinct enzymatic and nonenzymatic production pathways is an accurate, yet generalized, description of more complex and interrelated underlying processes. We organize epidemiologic evidence for oxylipins by biosynthetic pathway and by enzymatic or nonenzymatic production to accurately reflect important biological processes (Figure 1).
3. Circulating oxylipins and pregnancy outcomes
Our review of oxylipins and pregnancy outcomes focused on 17 studies of preeclampsia, 8 studies of preterm birth, and 8 studies of fetal growth (Figure 2).
Figure 2.
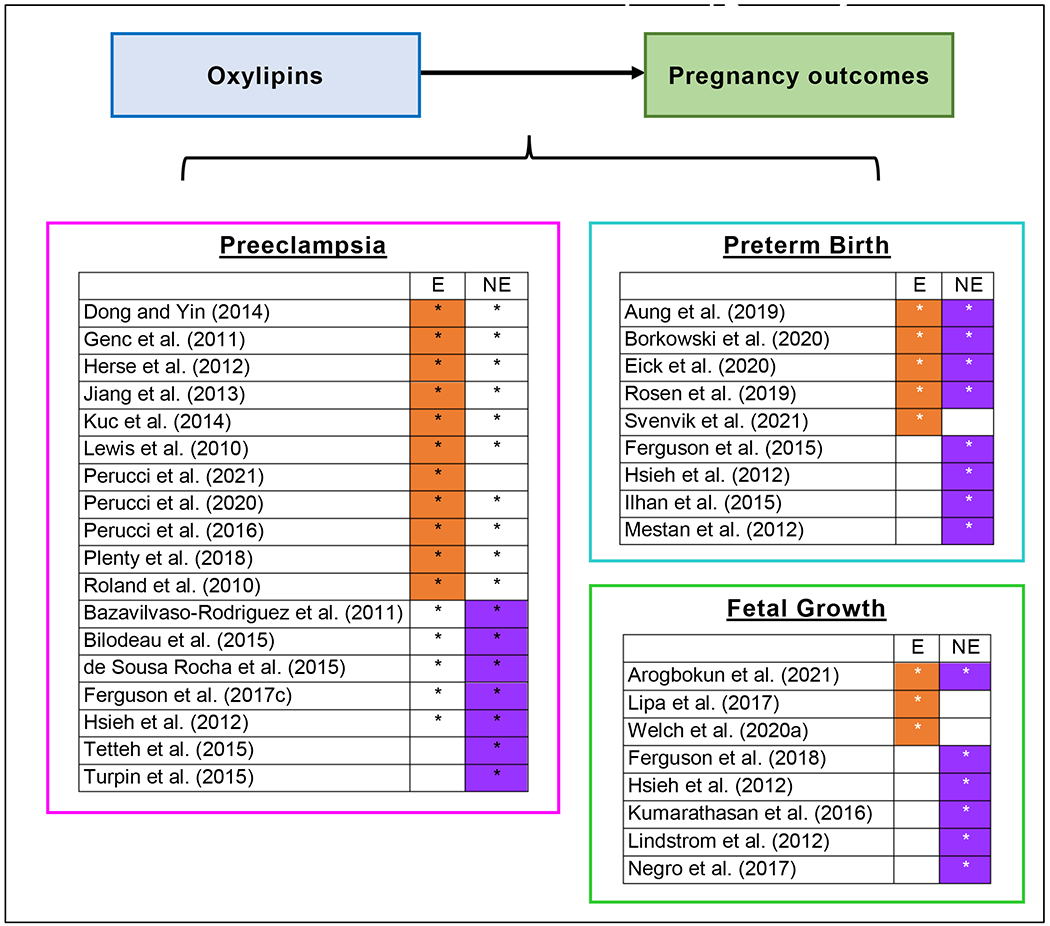
Overview of epidemiologic studies investigating associations between circulating oxylipins and pregnancy outcomes
Study evaluated oxylipins produced via enzymatic (E) and/or nonenzymatic (NE) biosynthetic pathways
3.1. Preeclampsia
Preeclampsia is a hypertensive disorder of pregnancy that presents serious risks to maternal and child health. Globally, preeclampsia remains a leading cause of maternal mortality and morbidity that impacts roughly 2% to 8% of pregnancies (Abalos et al., 2013). Over the past 30 years there has been a promising decrease in preeclampsia incidence due to improved health access and education, but a large disparity in occurrence still exists within and between countries (Wang et al., 2021b). Preeclampsia is generally defined by the onset of hypertension and excess proteinuria in pregnancy (ACOG 2020). The pathophysiological mechanisms leading to preeclampsia are multifactorial and remain largely uncertain, but robust evidence points towards a central role for dysregulated responses to inflammation and oxidative stress (Harmon et al., 2016). Epidemiologic evidence of inflammatory responses using measures of circulating oxylipins provides a promising avenue to improve understanding of underlying factors for preeclampsia. As summarized in Table 1, we identified 18 epidemiologic studies of preeclampsia and circulating oxylipins. Most studies were cross-sectional and measured only one or two individual oxylipin analytes from a single biosynthetic pathway.
Table 1.
Epidemiologic studies investigating associations between circulating oxylipins in pregnancy and preeclampsia (PE)
| Referencea | Country | Study design and sample size | Timingb | Matrix | Pathway: Oxylipins | PE associationsc |
|---|---|---|---|---|---|---|
| Dong and Yin (2014) | China | Design: Cross-sectional case-control Sample: N=90; n=60 PE |
Delivery | Cord blood serum | AA-LOX: LXA4 | (+) LXA4 |
| Genc et al. (2011) | Turkey | Design: Prospective case-control Sample: N=45; n=21 PE |
Trimesters 1-2 (2 samplings) | Serum | AA-COX: PGF2α | (+) PGF2α |
| Herse et al. (2012) | USA | Design: Case-control Sample: N=20; n=10 PE cases |
Not reported | Plasma | AA-CYP: EETs, DHETs, 20-HETE | (+) 5,6-(EET+DHET) (+) 14,15-(EET+DHET) |
| Jiang et al. (2013) | Italy | Design: Cross-sectional matched case-control Sample: N=48; n=19 PE cases |
Trimester 3 | Urine, plasma, and RBCs | AA-CYP: EETs, DHETs, 20-HETE | (−) DHET (urine) |
| Delivery | Plasma and RBC; Cord blood plasma and RBC | AA-CYP: EETs, DHETs, 20-HETE | ||||
| Kuc et al. (2014) | Netherlands | Design: Prospective case-control Sample: N=667; n=167 PE cases |
Trimester 1 | Serum | 46 oxylipins (analytes not reported) | |
| Lewis et al. (2010) | USA | Design: Cross-sectional case-control Sample: N=80; n=40 PE |
Delivery | Plasma | AA-COX: 6-keto-PGF1α, TXB2 | (−) 6-keto-PGF1α (−) ratio 6-keto-PGF1α:TXB2 |
| (Perucci et al., 2021) | Brazil | Design: Prospective Sample: N=28, n=11 PE cases | Trimesters 1-3 (3 samplings) | Plasma | AA-LOX: LTB4, LXA4, DHA-LOX: resolvin D1 |
(+) LTB4 (−) resolvin D1 |
| Perucci et al. (2020) | Brazil | Design: Cross-sectional matched case-control Sample: N=41; n=21 PE |
Trimester 3 | Plasma | AA-LOX: LTB4 DHA-LOX: resolvin D1, maresin 1 |
(+) LTB4 (−) resolvin D1 (−) ratio resolvin D1:LTB4 (−) ratio maresin 1:LTB4 |
| Perucci et al. (2016) | Brazil | Design: Cross-sectional case-control Sample: N=76, n=27 PE cases |
Trimester 3 | Plasma | AA-LOX: LXA4 | (+) LXA4 |
| Plenty et al. (2018) | USA | Design: Cross-sectional case-control Sample: N=18; n=9 PE cases |
Delivery | Plasma | AA-CYP: EETs, 20-HETE | (−) EET (+) ratio 20-HETE:total EET |
| Roland et al. (2010) | Canada | Design: Cross-sectional case-control Sample: N=59; n=29 PE |
Delivery | Plasma | AA-COX: 6-keto-PGF1α, TXB2 | (+) 6-keto-PGF1α (−) TXB2 (+) ratio 6-keto-PGF1α:TXB2 |
| Bazavilvaso-Rodriguez et al. (2011) | Mexico | Design: Cross-sectional case-control Sample: N=45; n=20 PE |
Trimester 3 | Serum | AA-nonenzymatic: 8-isoprostane | (+) 8-isoprostane |
| Bilodeau et al. (2015) | Canada | Design: Prospective case-control Sample: N=93; n=33 PE |
Trimesters 1-2 | Plasma | AA-nonenzymatic: Class III F2-isoprostane regioisomers (8-isoprostane, 8-iso-15-PGF2α); Class VI F2-isoprostane isomer sums (5-iso-PGF2α-VI) | (+) Class VI F2-isoprostanes |
| de Sousa Rocha et al. (2015) | Brazil | Design: Cross-sectional matched case-control Sample: N=36; n=18 PE |
Trimester 3 | Urine (24-hr sampling) | AA-nonenzymatic: 8-isoprostane | (−) 8-isoprostane |
| Ferguson et al. (2017c) | USA | Design: Prospective cohort Sample: N=441; n=50 |
Trimesters 1-3 (4 samplings) | Urine | AA-nonenzymatic: 8-isoprostane | (+) 8-isoprostane |
| Hsieh et al. (2012) | Taiwan | Design: Cross-sectional cohort Sample: N=503; n=10 PE |
Trimester 2 | Plasma | AA-nonenzymatic: 8-isoprostane | (+) 8-isoprostane |
| Tetteh et al. (2015) | Ghana | Design: Cross-sectional case-control Sample: N=102 PE (early vs late onset) |
Trimester 3 | Urine | AA-nonenzymatic: 8-isoprostane | (+) 8-isoprostane (early vs late PE) |
| Turpin et al. (2015) | Ghana | Design: Cross-sectional matched case-control Sample: N=150; n=50 PE |
Trimester 2 | Plasma | AA-nonenzymatic: 8-isoprostane | (+) 8-isoprostane |
Abbreviations: (−), negative association; (+), positive association; 8-isoprostane, 8-iso-prostaglandin-F2α; AA, arachidonic acid; COX, cyclooxygenase; CYP, cytochrome P450; DHA, docosahexaenoic acid; DHET, dihydroxy-eicosatrienoic acid; EET, epoxyeicosatrienoic acid; HETE, hydroxyeicosatetraenoic acid; LOX, lipoxygenase; LT, leukotriene; LX, lipoxin; PG, prostaglandin; RBC, red blood cell; TX, thromboxane
Reference studies were identified in PubMed using a multi-stage approach. First, the following search phrase was used to identify an initial list of human studies published since 2010: (preeclampsia) AND (oxylipin OR eicosanoid OR bioactive lipid OR isoprostane) AND (inflammation OR oxidative stress) AND (2010/1/1:2022/02/01 [pdat]) AND (humans[Filter]) AND (english[Filter]) NOT (review[Filter] OR systematicreview[Filter]). This search produced 54 results, which were then paired down to the 18 reviewed studies of fetal growth after excluding duplicates and reviewing articles for relevant areas of focus.
Single spot sample collected unless otherwise noted
Null associations left blank
Associations with enzymatic oxylipins:
Several studies investigated oxylipins produced from AA by CYP enzymes (AA-CYP). A prospective case-control study conducted in the US collected repeated plasma samples and observed a positive association between total EET and dihydroxy-eicosatrienoic acid (DHET) oxylipins and preeclampsia (Herse et al., 2012). Although EET has anti-inflammatory effects, elevated EET concentrations could indicate compensatory mechanisms to address systemic inflammation. Another cross-sectional study conducted among women at delivery observed opposing associations, as preeclampsia was associated with lower plasma EET concentrations (Plenty et al., 2018). Additionally, a cross-sectional study that collected fasting samples of urine, plasma, and red blood cells in the third trimester found inverse associations between urinary DHETs and preeclampsia, while null associations were observed for plasma and red blood cell concentrations of EETs, DHETs, and 20-HETE (Jiang et al., 2013). However, this study is difficult to compare with others as it assessed esterified oxylipins by collecting fasting samples (Jiang et al., 2013).
More consistent findings were observed between studies investigating oxylipins produced from AA by LOX (AA-LOX). Two cross-sectional case-control studies conducted in late pregnancy or at delivery both observed positive associations between preeclampsia and levels of lipoxin A4 (LXA4) in cord blood serum (Dong and Yin, 2014) or maternal plasma (Perucci et al., 2016). Similar to EETs, LXA4 has anti-inflammatory effects (Serhan, 2014), which may be upregulated to counteract pro-inflammatory processes of preeclampsia. Additionally, cross-sectional (Perucci et al., 2020) and longitudinal (Perucci et al., 2021) investigations in Brazil found a positive associations with LTB4, a pro-inflammatory AA-LOX oxylipin. However, these studies also showed that oxylipins produced by DHA-LOX with pro-resolving properties, including resolvin D1 and maresin 1, were lower in preeclamptic than normotensive pregnancies (Perucci et al., 2020; Perucci et al., 2021). Together, these findings indicate there may be relatively consistent processes involving anti-inflammatory oxylipins produced from AA-LOX with preeclampsia.
The other enzymatically produced oxylipins findings included those biosynthesized from AA by COX (AA-COX). We identified three studies, including one case-control study that evaluated early pregnancy serum samples (Gene et al., 2011) and two cross-sectional studies were identified that collected maternal plasma samples in late pregnancy or at delivery (Roland et al., 2010; Perucci et al., 2020). The case-control study observed positive prospective associations between prostaglandin F2α (PGF2α) and preeclampsia (Gene et al., 2011), but associations were complicated by the fact that serum samples could have elevated ex vivo formation of prostaglandins (Rund et al., 2020). The two cross-sectional studies investigated both 6-keto-prostaglandin F2α (6-keto-PGF2α) and thromboxane B2 (TXB2), which are stable indicators of the vasodilator prostacyclin (PGI2) and vasoconstrictor TXA2, respectively (Roland et al., 2010; Perucci et al., 2020). These studies observed preeclampsia associations in opposing directions for both oxylipins (Roland et al., 2010; Perucci et al., 2020). The conflicting, yet significant, results indicate that these important indicators of AA-COX activity should be investigated further in future studies.
Associations with 8-isoprostane:
Approximately half of the studies we evaluated performed analyses of F2-isoprostanes, which are oxylipins primarily produced from oxidative stress (i.e., the nonenzymatic peroxidation of AA). However, as previously noted, it is possible that low levels of 8-isoprostane in circulation are partially derived from COX (van ’t Erve et al., 2015). Regardless of their origin, prostaglandin-like molecules are considered to be both biomarkers and biological mediators of oxidative stress (Milne et al., 2015). Overall, most studies observed positive associations between F2-isoprostanes and preeclampsia, particularly for 8-isoprostane (Bazavilvaso-Rodriguez et al., 2011; Hsieh et al., 2012; Bilodeau et al., 2015; Tetteh et al., 2015; Turpin et al., 2015; Ferguson et al., 2017c), although one study found a protective effect (de Sousa Rocha et al., 2015). Most of the studies we evaluated point towards early pregnancy as a particularly critical period of pregnancy for potential effects of F2-isoprostanes on preeclampsia.
Importantly, there may also be certain phenotypes of preeclampsia that drive differences in inflammation and oxidative stress (Yagel et al., 2021). For example, the large prospective study by Ferguson et al. (2017c) observed that the association between 8-isoprostane and preeclampsia was driven by preeclamptic participants who did not deliver preterm. This finding indicated that oxidative stress plays a larger role for less severe phenotypes of preeclampsia (i.e., those with onset later in pregnancy) (Ferguson et al., 2017c). Additionally, poor placentation is typically considered a primary factor driving certain preeclampsia phenotypes (Steegers et al., 2010). Several studies have shown that placental tissue from preeclamptic pregnancies has higher concentrations of AA-CYP oxylipins compared to tissue from uncomplicated pregnancies (Pearson et al., 2010; Herse et al., 2012; Dalle Vedove et al., 2016; Plenty et al., 2018), and that differences are less apparent when examining levels in maternal plasma (Herse et al., 2012; Plenty et al., 2018). Thus, circulating oxylipin production may be less physiologically relevant to production levels in the placenta for certain preeclampsia phenotypes and these condition-specific considerations should be carefully evaluated when designing new studies.
3.2. Preterm birth
Preterm birth is broadly defined as delivering before 37 weeks of gestation (ACOG 2016). Globally, preterm birth impacts over 10% of pregnancies and is a leading cause of neonatal mortality and morbidity (Blencowe et al., 2013). Underlying risk factors for most preterm births are still uncertain, but increasing evidence supports a central role for dysregulated inflammation and oxidative stress (Cappelletti et al., 2016). Importantly, there is growing recognition that the distinct pathologies leading to preterm birth can produce disparate inflammatory conditions leading up to delivery (McElrath et al., 2008; Voltolini et al., 2013).
Oxylipins have garnered substantial clinical and scientific interest for playing a central role in parturition, which has predominately focused on the AA-COX-derived prostaglandins. Clinical administration of prostaglandins can induce uterine contractions and labor, while inhibition of prostaglandin biosynthesis can prevent or prolong labor and delivery (Alfirevic et al., 2015). Prostaglandins such as PGE2 and PGF2α are well recognized to play a role in parturition, as elevated levels can stimulate uterine contractions and promote cervix dilation and thinning through coordinated inflammatory responses (Olson and Ammann, 2007). However, our review highlights the expanding epidemiologic literature which suggests that preterm delivery is associated with dysregulation of a wider array of oxylipins. We identified 9 epidemiologic studies of circulating oxylipins in pregnancy and preterm birth (Table 2). Most studies had prospective study designs but were limited by the collection of a single maternal sample prior to delivery.
Table 2.
Epidemiologic studies investigating associations between circulating oxylipins in pregnancy and preterm birth (PTB)
| Referencea | Country | Study design and sample size | Timingb | Matrix | Oxylipins measured | PTB associationsc |
|---|---|---|---|---|---|---|
| Aung et al. (2019) | USA | Design: Prospective case-control Sample: N=173; n=58 PTB (spontaneous, placental) |
Trimester 2 | Plasma, urine | 48 oxylipins in plasma (all major enzymatic pathways) AA-nonenzymatic: 8-isoprostane (urine, total) | (+) LA-CYP/LOX (+/−) AA-CYP (+) AA-LOX/COX (+) 8-isoprostane |
| Borkowski et al. (2020) | USA | Design: Prospective matched case-control Sample: N=102; n=51 PTB (<31 weeks) |
Trimester 2 | Serum | 82 oxylipins (all major enzymatic pathways; AA- nonenzymatic pathway) | (+) AA-CYP/LOX/COX oxylipins (+) LA-CYP/LOX oxylipins (+) F2-isoprostanes |
| Eick et al. (2020) | Puerto Rico |
Design: Prospective Sample: N=469, n=50 PTB |
Trimesters 2-3 (3 samplings) | Urine | AA-COX: PGF2α AA-nonenzymatic: 8-isoprostane (free), 8-isoprostane metabolite |
(+) 8-isoprostane (+) 8-isoprostane metabolite (+) PGF2α |
| Rosen et al. (2019) | USA | Design: Prospective Sample: N=740; n=61 PTB (overall, spontaneous) |
Trimester 3 | Urine | AA-COX: PGF2α AA-nonenzymatic: 8-isoprostane (free), 8-isoprostane metabolite |
(+) 8-isoprostane metabolite |
| (Svenvik et al., 2021) | Sweden | Design: Cross-sectional (PTB-only) Sample: N=80; n=21 PTB (<34 weeks) |
Delivery or Trimester 3 | Plasma | 67 oxylipins (all major enzymatic pathways) | (−) 9,10-DiHOME (LA-LOX) and 9,10-DiHODEs (αLA-LOX) (+) 8-HETE (AA-LOX) |
| Ferguson et al. (2015) | USA | Design: Prospective case-control Sample: N=482; n=130 PTB (spontaneous, placental) |
Trimesters 1-3 (4 samplings) | Urine | AA-nonenzymatic: 8-isoprostane (total) | (+) 8-isoprostane |
| Hsieh et al. (2012) | Taiwan | Design: Prospective Sample: N=503; n=17 PTB |
Trimester 2 | Plasma | AA-nonenzymatic: 8-isoprostane | |
| Ilhan et al. (2015) | Turkey | Design: Cross-sectional matched case-control Sample: N=72*, n=34 PTB * |
Delivery | Plasma | AA-nonenzymatic: 8-isoprostane | (−) 8-isoprostane |
| Mestan et al. (2012) | USA | Design: Cross-sectional Sample: N=237 PTB (≤28, 29-33, and <37 weeks) |
Delivery | Cord blood plasma | AA-nonenzymatic: 8-isoprostane | (+) 8-isoprostane (early vs late PTB) |
Abbreviations: 8-isoprostane, 8-iso-prostaglandin-F2α; AA, arachidonic acid; COX, cyclooxygenase; CYP, cytochrome P450; LA, linoleic acid; LOX, lipoxygenase; PG, prostaglandin
Reference studies were identified in PubMed using a multi-stage approach. First, the following search phrase was used to identify an initial list of human studies published since 2010: (preterm OR gestational age) AND (oxylipin OR eicosanoid OR bioactive lipid OR isoprostane) AND (inflammation OR oxidative stress) AND (2010/1/1:2022/02/01[pdat]) AND (humans[Filter]) AND (english[Filter]) NOT (review[Filter] OR systematicreview[Filter]). This search produced 145 results, which were then paired down to the 9 reviewed studies of preterm birth after excluding duplicates and reviewing articles for relevant areas of focus.
Single spot sample collected unless otherwise noted
Null associations left blank
Associations with enzymatic oxylipins:
Three studies quantified a robust set of approximately 50-80 oxylipins that fell along all major biosynthetic pathways (i.e., LA, AA, DHA, and EPA precursors with nonenzymatic or CYP, LOX, COX production). This included a case-control study by Borkowski et al. (2020) of maternal serum collected in the early second trimester (15-17 weeks). Borkowski et al. (2020) found that oxylipins from nearly all major biosynthetic pathways were positively associated overall preterm birth (Borkowski et al., 2020). However, these associations were observed almost exclusively among women with prepregnancy obesity, had limited covariate adjustments (maternal age and race/ethnicity), and had limited sample sizes for preterm birth (n=17) (Borkowski et al., 2020). Another case-control study by Aung et al. (2019) examined a large panel of oxylipins in maternal plasma and urine collected in the late second trimester (23-28 weeks). The authors also observed positive associations between oxylipins from several biosynthetic pathways with preterm birth, particularly for oxylipins produced from AA or LA precursors by LOX enzymes (Aung et al., 2019), after accounting for several important covariates and in a sample that included a larger number of preterm births (n=58). Finally, a cross-sectional study in Sweden evaluated a large number of plasma oxylipins on women at high risk of preterm delivery who presented symptoms of delivery onset at study enrollment (Svenvik et al., 2021). Although this study found interesting associations with several biosynthetic pathways, the results are difficult to compare with other studies because preterm birth was defined as delivery before 34 weeks gestation and samples were taken at different gestational windows depending on preterm outcome (Svenvik et al., 2021).
Associations with 8-isoprostane:
As with studies of preeclampsia, the majority of the research on oxylipins and preterm birth focused on associations with oxidative stress using 8-isoprostane. The strongest study design was a large case-control study that collected repeated urine samples spanning all trimesters (Ferguson et al., 2015). The authors observed positive associations between urinary 8-isoprostane and preterm birth, which were most evident among samples collected in late pregnancy (Ferguson et al., 2015). These findings were consistent with a prospective cohort study conducted in Puerto Rico, which observed 8-isoprostane, its primary metabolite, and the AA-COX produced oxylipin PGF were positively associated with preterm birth, and that associations were strongest in late pregnancy (Eick et al., 2020). Similarly, Rosen et al. (2019) observed positive associations between urinary levels of the primary metabolite of 8-isoprostane measured in late pregnancy and preterm birth, though they did not observe associations with the parent compound. Other studies that measured 8-isoprostane in plasma rather than urine, at mid-pregnancy or at delivery, found inconsistent associations with preterm birth (Hsieh et al., 2012; Mestan et al., 2012; Ilhan et al., 2015). Studies of amniotic fluid (Longini et al., 2007; Lee et al., 2008; Park et al., 2016), umbilical cord tissue (Hong et al., 2016), and placental tissue (Mitchell et al., 2016) have also had varying results.
The discrepancies in associations could be attributable to the heterogeneity in the tissue and gestational timing of sample collection. However, as with preeclampsia, heterogeneity in findings for preterm birth could also be due to distinct outcome subtypes, which were evaluated in several of the studies we evaluated (Ferguson et al., 2015; Aung et al., 2019; Rosen et al., 2019). The study of mid-pregnancy oxylipins by Aung et al. (2019) observed that associations between oxylipins and preterm birth were primarily driven by participants who experienced a spontaneous preterm birth. The study by Ferguson et al. (2015) of 8-isoprostane, which was within the same hospital-based pregnancy cohort, also observed that associations were driven by spontaneous preterm birth. However, Rosen et al. (2019) did not observe evidence of differences in associations between urinary 8-isoprostane and preterm birth by subtype. Additional investigations are needed to help determine whether certain subtypes of preterm birth are more susceptible to dysregulated oxylipin production.
3.3. Fetal growth
Fetal growth disorders are major risk factors for adverse maternal and fetal pregnancy outcomes (McCormick, 1985; Oral et al., 2001). Lower weight for gestational age at birth is associated with complications that have persistent effects across the life course (Barker, 2006). Clinical definitions of fetal growth restriction or excessive fetal growth often vary between studies, but are typically based on standardized measures of estimated fetal weight, birth weight, or other anthropometric measures (e.g., abdominal or head circumference) taken at birth or in utero by ultrasound (ACOG 2021). The most common measures include small for gestational age (SGA) and large for gestational age (LGA), which are generally used to describe newborns with birth weight that is <10th percentile or >90th percentile for gestational age, respectively. The primary pathological processes underlying fetal growth disorders often involve poor uterine-placental perfusion (ACOG 2021), but the etiology in most cases is still uncertain (Mandy et al., 2019). However, increasing evidence shows subclinical inflammation and oxidative stress may play a crucial role in fetal development (Ernst et al., 2011; Thompson and Al-Hasan, 2012). We identified 8 epidemiologic studies that evaluated circulating maternal oxylipins in pregnancy and fetal growth outcomes (Table 3), most of which evaluated prospective associations between oxylipins and fetal size at birth.
Table 3.
Epidemiologic studies investigating associations between circulating oxylipins in pregnancy and fetal growth
| Referencea | Country | Growth measure outcomes | Study design and sample size | Sample timingb | Sample Matrix | Oxylipins measured | Fetal growth associationc |
|---|---|---|---|---|---|---|---|
| Arogbokun et al. (2021) | USA | Birth weight for gestational age (sex specific) | Design: Prospective Sample: N=736 |
Trimester 3 | Urine | AA-COX: PGF2α AA-nonenzymatic: 8-isoprostane (free), 8-isoprostane metabolite | Birth weight:(−) 8-isoprostane and metabolite |
| Lipa et al. (2017) | Poland | SGA (≤10th percentile for gestational age) LGA (≥90th percentile for gestational age) |
Design: Prospective case-control Sample: N=185; n=19 SGA; n=32 LGA |
Trimester 1-2 | Serum | AA-LOX: LXA4 | SGA and LGA: (−) LXA4 |
| Welch et al. (2020a) | USA | SGA (≤10th percentile for gestational age) LGA (≥90th percentile for gestational age) |
Design: Prospective matched case-control Sample: N=90; n=31 SGA; n=28 LGA |
Trimesters 1-3 (3 samplings) | Plasma | 57 oxylipins (all major enzymatic pathways) | SGA: (+) LA-LOX (9- & 13-HODE) (+) AA-CYP (DHETs, 19- & 20-HETE) (+) AA-LOX (5-, 8-, 12-, & 15-HETE) |
| Ferguson et al. (2018) | USA | Ultrasound: head circumference, abdominal circumference, femur length, estimated fetal weight Delivery: birthweight for gestational age |
Design: Prospective Sample: N=482 |
Trimesters 1-3 (4 samplings) | Urine | AA-nonenzymatic: 8-isoprostane (total) | Birth weight: (−) 8-isoprostane Head and femur circumference (ultrasound): (−) 8-isoprostane |
| Hsieh et al. (2012) | Taiwan | SGA (≤10th percentile for gestational age and sex) | Design: Prospective Sample: N=503; n=40 SGA |
Trimester 2 | Plasma | AA-nonenzymatic: 8-isoprostane | SGA: (+) 8-isoprostane |
| Kumarathasan et al. (2016) | Canada | Birth weight (categorical quantiles at <25th, 25-75th, >75th) | Design: Prospective Sample: N=144 |
Trimester 3 | Plasma | AA-nonenzymatic: 8-isoprostane | Birth weight (<25th percentile): (−) 8-isoprostane |
| Lindstrom et al. (2012) | Bangladesh | Low birth weight (<2500 g), low birth length (lowest tertile), small birth head (lowest tertile), small birth chest circumference (lowest tertile) | Design: Prospective Sample: N=374 |
Trimester 2-3 (2 samplings) | Urine | AA-nonenzymatic: 8-isoprostane | Low birth weight, small length, small chest circumference LBW, small birth length, small birth chest circumference: (+) 8-isoprostane (middle tertile) |
| Negro et al. (2017) | Italy | SGA LGA |
Design: Cross-sectional matched case-control Sample: N=245 |
Delivery | Cord blood plasma | AA-nonenzymatic: 8-isoprostane | SGA: (+) 8-isoprostane |
Abbreviations: 8-isoprostane, 8-iso-prostaglandin-F2α; AA, arachidonic acid; COX, cyclooxygenase; CYP, cytochrome P450; DHET, dihydroxy-eicosatrienoic acid; HETE, hydroxyeicosatetraenoic acid; HODE, hydroxy-octadecadienoic acid; LGA, large for gestational age; LOX, lipoxygenase; LX, lipoxin; SGA, small for gestational age
Reference studies were identified in PubMed using a multi-stage approach. First, the following search phrase was used to identify an initial list of human studies published since 2010: (IUGR OR fetal growth OR SGA) AND (oxylipin OR eicosanoid OR bioactive lipid OR isoprostane) AND (inflammation OR oxidative stress) AND (2010/1/1:2022/02/01 [pdat]) AND (humans[Filter]) AND (english[Filter]) NOT (review[Filter] OR systematicreview[Filter]). This search produced 78 results, which were then paired down to the 8 reviewed studies of fetal growth after excluding duplicates and reviewing articles for relevant areas of focus.
Single spot sample collected unless otherwise noted
Null associations left blank
Associations with enzymatic oxylipins:
The most comprehensive study evaluated repeated measures of oxylipins from all major biosynthetic pathways using a case-control study design of SGA cases, LGA cases, and controls (Welch et al., 2020a). Pro-inflammatory plasma oxylipins produced by the oxidation of AA by CYP (i.e., DHETs, 19-/20-HETEs) or LOX (i.e., HETEs) enzymes, as well as those produced from LA by LOX (i.e., 9-/13-hydroxy-octadecadienoic acids [HODEs]) enzymes, were higher among participants who went on to deliver SGA infants (Welch et al., 2020a). However, associations were mostly null for participants who delivered LGA infants (Welch et al., 2020a). Importantly, this study provided novel evidence of important non-linear changes in circulating oxylipin levels across pregnancy, regardless of the infant size at delivery (Welch et al., 2020a). Another case-control study conducted spot serum sampling in the late first trimester to early second trimester and found LXA4, a pro-resolving and anti-inflammatory oxylipin produced from AA by LOX, was lower in participants who went on to delivery SGA or LGA compared to controls (Lipa et al., 2017). Additional studies are needed to confirm findings from these two initial studies, but these results support the hypothesis that abnormal fetal growth outcomes may be influenced by dysregulated enzymatic oxylipin production.
Associations with 8-isprostane:
Similar to preeclampsia and preterm birth, the majority of studies we identified focused on 8-isoprostane. Overall, despite the large variability in study designs, these studies had remarkable consistency in results that showed higher 8-isoprostane in maternal circulation is associated with reduced fetal growth. Only one study conducted maternal plasma sampling across all trimesters alongside repeated in utero ultrasound measures of fetal growth (Ferguson et al., 2018), which may better capture true pathological abnormalities of fetal growth (ACOG 2021). This study found inverse associations between urinary 8-isoprostane and fetal growth (Ferguson et al., 2018), with the strongest associations observed in the late second trimester and for measures of head circumference and femur length (Ferguson et al., 2018). Another study of third trimester urine samples also found that the primary metabolite of 8-isoprostane was associated with reduced size for gestational age (Arogbokun et al., 2021). Finally, Hsieh et al. (2012) showed an association between 8-isoprostane and SGA which was independent of preeclampsia status.
4. Oxylipins as biological mediators of environmental exposures in pregnancy
Environmental exposures are important risk factors for disease across the life course. Pregnancy represents a particularly critical period for the adverse effects of environmental exposures for both maternal and fetal health (Varshavsky et al., 2020). While there is growing recognition that chemical exposures play a part in a range of adverse pregnancy processes, the understanding of mechanisms connecting exposure to health outcomes remains limited. For many chemical exposures, existing evidence supports the hypothesis that dysregulated inflammation and oxidative stress processes play an important role (Kahn and Trasande, 2018). Investigating oxylipins in the continuum between environmental exposures and pregnancy outcomes may improve our understanding of the toxicity of these compounds and provide opportunities to mitigate adverse effects. We identified three general categories of environmental exposures that have been evaluated for associations with circulating oxylipins in pregnancy (Figure 3), including consumer product chemicals, heavy metals, and air pollutants, including particulate matter (PM) and polycyclic aromatic hydrocarbons (PAHs).
Figure 3.
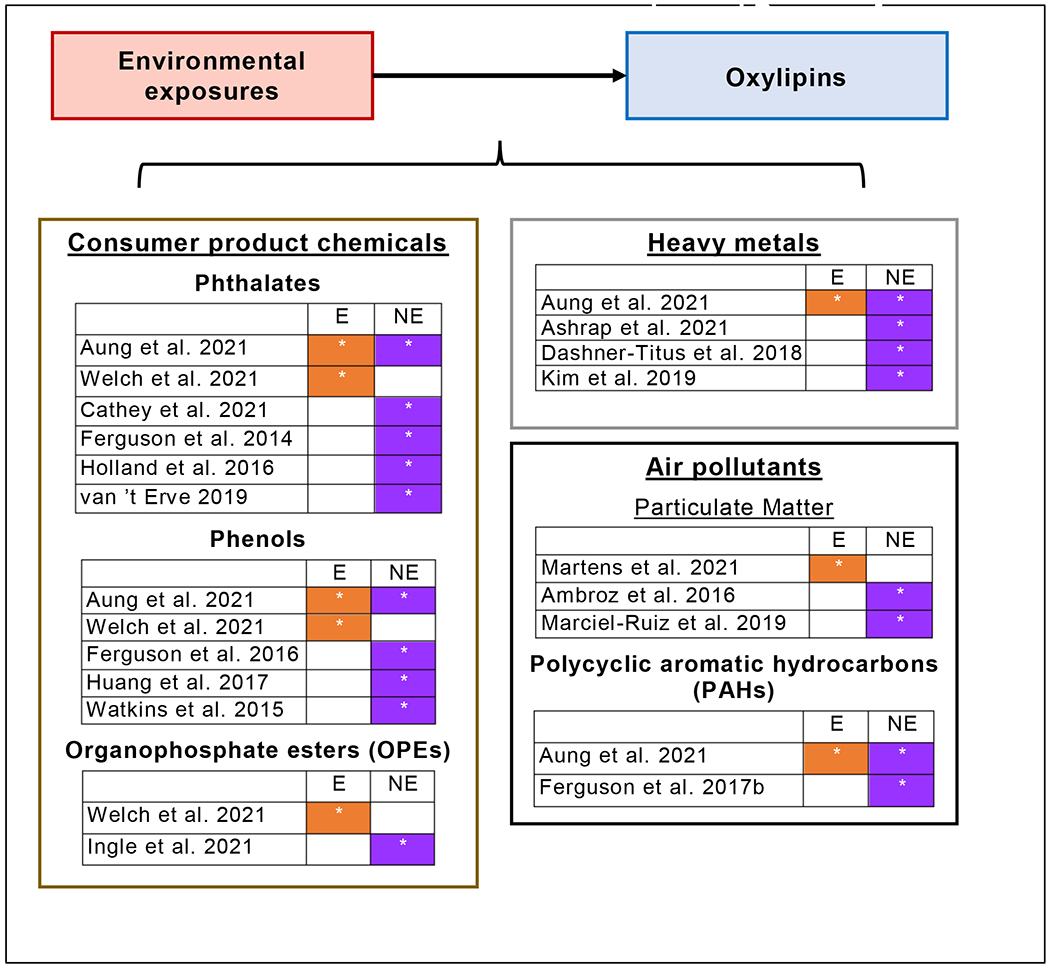
Overview of epidemiologic studies investigating associations between circulating oxylipins in pregnancy and environmental exposures
Study evaluated oxylipins produced via enzymatic (E) and/or nonenzymatic (NE) biosynthetic pathways.
4.1. Consumer product chemicals
The largest proportion of studies evaluated exposure to synthetic chemicals in consumer products, including phthalates, phenols, and organophosphate esters (OPEs). Each of these classes of consumer product chemicals have the capacity to act as endocrine disruptors and are heavily used across the globe, including among pregnant populations (Woodruff et al., 2011; Zoeller et al., 2012). Additionally, higher concentrations of exposure biomarkers to these chemicals in pregnancy have been associated with preeclampsia (Rosen et al., 2018), preterm birth (Ferguson and Chin, 2017), and fetal growth (Kamai et al., 2019).
Associations with enzymatic oxylipins:
Two studies of oxylipins evaluated urinary biomarkers of both phthalates and phenols within separate subsets of the same pregnancy cohort in the US (Aung et al., 2021; Welch et al., 2021). While these studies had different study designs and statistical approaches, both evaluated plasma oxylipins produced along each major enzymatic pathway. The longitudinal study by Welch et al. (2021) found that mixtures of urinary phenols measured across pregnancy were positively associated with pro-inflammatory oxylipins produced by LA-LOX (i.e., HODEs) and AA-LOX (i.e., HETEs), while mixtures of urinary phthalate biomarkers were positively associated with LA-CYP oxylipins (i.e., epoxy-octadecenoic acid [EpOME], dihydroxy-octadecenoic acid [DiHOME]). Further, this study provided novel evidence that OPE mixtures were positively associated with LA-CYP oxylipins (Welch et al., 2021). The cross-sectional study conducted at mid-pregnancy by Aung et al. (2021) observed generally similar associations between individual oxylipins and urinary phenol or phthalate biomarkers, but found stronger associations with anti-inflammatory AA-CYP oxylipins (i.e., EETs). Together, these findings provide evidence that each chemical class may act upon specific biosynthetic pathways of oxylipin production.
Associations with 8-isoprostane:
All other studies of phthalates or phenols we evaluated had a primary focus on associations with 8-isoprostane levels. Regardless of study design or approach, the studies of urinary phthalate biomarkers found positive associations with 8-isoprostane (Ferguson et al., 2014a; Holland et al., 2016; van’t Erve 2019; Cathey et al., 2021). Interestingly, the study by van’t Erve et al. (2019) observed that the nonenzymatic fraction of 8-isoprostane produced via oxidative stress drove most of the associations, and that participants who reported using dietary supplements high in omega-3 PUFAs had associations of lower magnitude. The studies evaluating urinary phenols or OPEs all observed positive associations with urinary 8-isoprostane (Watkins et al., 2015; Ferguson et al., 2016; Huang et al., 2017; Ingle et al., 2020). These lines of evidence for may point towards a particularly important role for consumer product chemicals at initiating nonenzymatic oxylipin production and oxidative stress.
4.2. Heavy metals
Exposure to heavy metals (e.g., arsenic, cadmium, and lead) is well recognized to influence reproductive health and children’s development (Rehman et al., 2018). There is also increasing evidence that heavy metals can impair early life immune function and instigate prolonged dysregulation of inflammation and oxidative stress (Dietert, 2009; Welch et al., 2020b). However, evidence is limited regarding how oxylipins may be involved with these effects. We identified 4 studies that evaluated associations between metals and oxylipins in pregnancy.
Associations with enzymatic oxylipins:
The previously mentioned study by Aung et al. (2021) found negative associations between individual metals (e.g., barium, lead) and LA-LOX oxylipins (e.g., HODE metabolite). However, associations were less consistent between statistical models that evaluated metals individually or as a mixture (Aung et al., 2021).
Associations with 8-isoprostane:
Although studies focusing on 8-isoprostane found positive associations with nickel (Ashrap et al., 2021), copper (Kim et al., 2019), or arsenic (Dashner-Titus et al., 2018), heavy metals such as lead and cadmium showed poor consistency in associations with 8-isoprostane across studies. This is somewhat surprising, given that both lead and cadmium have had robust links to oxidative stress in previous studies (Patra et al., 2011). These inconsistencies may be due to differences in the distribution of metals exposures between these populations, but may also show that metals have greater relative impact on immunological processes that are independent of oxylipin production, such as redox signaling that primarily impacts proteins or DNA (Schieber and Chandel, 2014).
4.3. Air pollutants
Air pollution is a pervasive and growing threat to global public health (Cohen et al., 2017). Exposure to low levels of ambient air pollution in pregnancy is associated with a range of adverse outcomes, including preeclampsia (Yu et al., 2020), preterm birth (Hansen et al., 2006), and fetal growth restriction (Liu et al., 2007). Although air pollution is recognized to initiate a range of inflammatory reactions (Vawda et al., 2014), few studies have investigated how exposure may influence oxylipin production in pregnancy. We identified 5 studies that investigated prenatal oxylipin levels and air pollution based on exposure to particulate matter and polycyclic aromatic hydrocarbons (PAHs). Ambient airborne particulate matter is a primary indicator of air pollution and is composed of a complex mixture of small particles. PAHs are a diverse class of chemicals emitted from the combustion of a large variety of fuel sources (e.g., coal, gasoline, wood) and represent an important component of particulate matter.
Associations with enzymatic oxylipins:
An air pollution study conducted in Belgium found that pro-inflammatory oxylipins measured in cord blood were higher among individuals who experienced higher environmental exposure to particulate matter with aerodynamic diameter <2.5 μm (PM2.5) across pregnancy (Martens et al., 2017). The study observed that oxylipins produced by specific LOX isoforms, including 5-LOX and 12/15-LOX, were the primary drivers of positive associations, while mixtures of oxylipins produced by CYP or COX had null associations with PM2.5 (Martens et al., 2017). The potentially important role of LOX-derived oxylipins in response to small, aerosolized particle exposures has also been shown in epidemiologic studies of adults (Wang et al., 2021a) and experimental studies of mice (Li et al., 2015). The previously mentioned study by Aung et al. (2021) observed positive associations between mid-pregnancy urinary metabolites of PAH exposures and LOX-produced oxylipins.
Associations with 8-isoprostane:
The other studies of air pollution focused on oxylipins primarily produced by oxidative stress. Two studies of particulate matter exposure found null associations with maternal or cord blood 8-isoprostane and a related regioisomer (Ambroz et al., 2016; Maciel-Ruiz et al., 2019). However, a cross-sectional study found positive associations between maternal urinary PAHs and 8-isoprostane in samples collected at the beginning of the third trimester (Ferguson et al., 2017b). Given that particulate matter is measured in the environment and PAHs are typically measured in biospecimens like urine, divergent associations are not altogether surprising. Future studies could improve the literature by addressing what chemical components of particulate matter and PAH are driving associations with oxidative stress.
4.4. Oxylipins as mediators of associations between environmental exposures and pregnancy outcomes
An underlying hypothesis of this review is that oxylipins act as important biological mediators of the relationship between environmental exposures and pregnancy processes. Several initial studies have employed innovative study designs and statistical methods to evaluate how oxylipins may mediate the effect of environmental chemicals on pregnancy outcomes, primarily regarding preterm birth (Mustafa et al., 2015; Ferguson et al., 2017a; Aung et al., 2020). However, it is important to note that statistical mediation analyses of epidemiologic data require strong causal assumptions that can bias associations when not met (Vanderweele, 2015). We see three key areas in which future studies can provide a better foundation to understand the likelihood that certain causal assumptions are being met within observational settings.
First, there is a critical need for additional evidence focused on the mechanisms of toxicant actionon oxylipin production in pregnancy. This includes experimental evidence focused on understanding the biological mechanisms by which specific classes of environmental exposures may differentially modulate activity of the various enzymes that biosynthesize oxylipins. Further, additional epidemiologic evidence is needed to evaluate whether certain gestational periods are most susceptible to changes in oxylipin production in response to environmental exposures. Second, future studies should evaluate how critical modifiable factors may ameliorate effects of environmental exposures on oxylipin production in pregnancy. For example, several epidemiologic studies have evaluated how dietary indicators (e.g., omega-3 supplementation, PUFAs ratios in plasma) may impact associations between exposure biomarkers and circulating oxylipin levels in pregnancy (van’t Erve 2019; Welch et al., 2021). Such metrics can provide potential insights into biological mechanisms and at the same time help inform potential public health interventions. Third, future studies should carefully consider evaluating how mixtures of chemical exposures may be impacting oxylipin production in pregnancy. Although accounting for exposure mixtures can increase the cost and complexity of any epidemiologic study, it may provide much more translatable research that accounts for co-exposures to related environmental pollutants (Carlin et al., 2013). Several studies we included in our review employed innovative statistical approaches to account for chemical mixtures (Ferguson et al., 2019; Kim et al., 2019; Aung et al., 2021; Cathey et al., 2021; Welch et al., 2021). Given that some of these initial studies have shown that different classes of chemicals are differentially associated with distinct oxylipin pathways (Aung et al., 2021; Welch et al., 2021), additional evidence of pathway-specific effects is needed to better characterize expected impacts on inflammation. All of the recommended research areas we have highlighted will benefit from increased collaboration between molecular toxicologists, reproductive biologists, and epidemiologists.
5. Therapeutic options to regulate oxylipin production in pregnancy
The modulation of oxylipin production may provide a viable way to improve pregnancy health outcomes and ameliorate the effects of environmental exposures when avoiding exposure is challenging or impossible. Oxylipin production has been a longstanding target of pharmaceutical and therapeutic treatments. Many existing therapeutic interventions are aimed at resolving dysregulated inflammation by promoting or inhibiting the production of oxylipins (Panigrahy et al., 2021). The termination of pro-inflammatory processes can be promoted by a group of endogenously synthesized oxylipins called specialized pro-resolving mediators (SPMs), such as resolvins, maresins, protectins, and neuroprotectins (Serhan, 2014). However, most SPMs have not been investigated or identified in maternal circulation during pregnancy (Elliott et al., 2017). Here, we briefly review the most promising and extensively studied of these therapeutics, which include non-steroidal anti-inflammatory drugs (NSAIDs), dietary PUFA supplementation, and soluble epoxide hydrolase (sEH) inhibitors.
5.1. NSAIDs
An increasing number of medications aim to inhibit certain enzyme families that synthesize pro-inflammatory oxylipins while promoting the action of others that produce anti-inflammatory oxylipins. A wide array of non-steroidal anti-inflammatory drugs (NSAIDs; e.g., aspirin, celecoxibs, indomethacin) inhibit specific enzymes (e.g., COX isoforms) and can be prescribed in pregnancy (Strauss et al., 2018). Short-term administration of aspirin may be one of the safest and most practical drug therapies to ameliorate dysregulated oxylipin production in pregnancy. Aspirin and its salicylate derivates trigger the production of lipoxins with pro-resolving effects that have stronger bioactivity than when naturally produced from AA by LOX (e.g., LXA4) (Serhan, 2014). Low-dose aspirin prophylaxis is recommended for women at high risk of preeclampsia, with treatment initiation currently recommended between 12-28 weeks of gestation (ACOG 2018). However, meta-analysis results show that administration in early pregnancy (<16 weeks) is what can dramatically reduce preeclampsia risk, while later administration (≥16 weeks) may have minimal impacts (Roberge et al., 2012). This time-sensitive risk reduction suggests that aspirin’s pro-resolving and anti-inflammatory effects may be aiding the implantation process when administered prior to chorionic plate formation (Lipa et al., 2017). Additional studies are needed to determine how aspirin and related NSAIDs may impact maternal inflammation processes in pregnancies without high preexisting risk of preeclampsia. Further, there is still need for improved specificity of these medications for target enzyme isoforms, which can result in fewer adverse effects and reduce risks to fetal health (Strauss et al., 2018).
5.2. Dietary PUFA and antioxidant supplementation
Dietary supplementation with long-chained PUFAs is another intensively studied, safe, and practical intervention that aims to mitigate dysregulated oxylipin production (Djuricic and Calder, 2021). Evidence for the beneficial effects of DHA, an omega-3 PUFA, on newborn cognitive development is strong enough that DHA is a required additive for infant formula in Europe (EFSA 2014). Growing evidence from randomized control trials, including demonstrable changes of oxylipin levels in maternal circulation (Ramsden et al., 2020), supports a role for omega-3 supplementation in reducing risk of adverse maternal and fetal pregnancy outcomes (e.g., preeclampsia, preterm birth and SGA) (Vinding et al., 2019). For example, a randomized control trial showed that DHA supplementation during pregnancy was associated with larger infant size (weight, length, head circumference), as well as lower risk of preterm delivery (Carlson et al., 2013). However, there is still considerable heterogeneity in results across studies that have prevented any widespread recommendations about clinical use (Saccone et al., 2016). Given the multifaceted benefits of omega-3s on maternal and fetal health (Larque et al., 2012), there is a need for future clinical studies to more thoroughly investigate whether supplementation may reduce risk of certain outcomes. Additionally, observational studies can at least address whether dietary PUFA supplementation influences associations between oxylipins and pregnancy outcomes by using objective biomarker measures, such as the ratio of plasma omega-6 to omega-3 PUFAs (Simopoulos, 2016).
Along with modulating inflammation, dietary supplementation with omega-3 PUFAs has been shown to have secondary effects on oxidative stress (Oppedisano et al., 2020; Djuricic and Calder, 2021). However, other dietary antioxidant therapeutics are important to consider, including the well-recognized antioxidants vitamins E and C (Duhig et al., 2016). Vitamin C removes free radicals in the aqueous phase and vitamin E helps to mitigate lipid peroxidation (Traber and Stevens, 2011). An early, randomized control trial with relatively small sample size (N=283) of vitamin C and E supplementation in pregnancy showed treatment reduced incidence of preeclampsia (Chappell et al., 1999). While larger subsequent randomized control trials found that vitamin supplementation may improve antioxidant capacity in treatment groups (Poston et al., 2006), the overwhelming body of evidence shows these treatments do not prevent preeclampsia regardless of high-or low-risk status (Poston et al., 2006; Villar et al., 2009; Roberts et al., 2010; Tenorio et al., 2018). Additional work is needed to determine whether certain combinations of antioxidants and PUFA supplementation could provide effective means to reduce oxidative stress the risk of pregnancy complications.
5.3. Soluble epoxide hydrolase inhibitors
Epoxygenated oxylipins produced by CYP enzymes (e.g., EETs, EpOMEs) have strong anti-inflammatory bioactivity (Dennis and Norris, 2015). The most extensively studied epoxygenated oxylipins are the AA-derived EETs. The anti-inflammatory and vasodilatory properties of EETs provide cardioprotective effects (Spector et al., 2004). However, these effects are often limited because EETs are rapidly hydrolyzed to their less-active diol forms (DHETs) by soluble epoxide hydrolases (sEH) (Dennis and Norris, 2015). This has made inhibition of sEH activity a longstanding target for pharmaceutical therapies (Imig and Hammock, 2009). Clinical and pharmaceutical studies have found promising beneficial effects of sEH inhibition on a range of cardiovascular conditions, including dysregulated inflammation and hypertensive disorders (Shen and Hammock, 2012). Further, sEH inhibition may provide benefits outside of increasing EET bioactivity. For example, the CYP subfamilies CYP2C and CYP2J that produce EETs also metabolize LA to form EpOME, which can be hydrolyzed to the corresponding diol DiHOME by epoxide hydrolases (Dennis and Norris, 2015). DiHOMEs are produced in much higher abundance throughout the body than EETs and, importantly, have opposing pro-inflammatory and vasoconstrictive effects (Figure 1) (Edin et al., 2020). Thus, carefully designed sEH inhibitors could potentially produce a range of beneficial effects by increasing the activity of anti-inflammatory oxylipins from several biosynthetic pathways (Norwood et al., 2010). It is unclear what role genetic polymorphisms may play at predisposing individuals to pregnancy complications by altering oxylipin production from CYP or sEH activity. Prior studies of preeclampsia or renovascular hypertension found null associations between pregnancy conditions and CYP or sEH polymorphisms (Bellien and Joannides, 2013). However, understanding these and other genetic polymorphisms as they relate to oxylipin activity and pregnancy outcomes may be an interesting direction for future research. While sEH activity has been minimally studied in pregnancy, increasing evidence points towards a role in pregnancy outcomes such as preeclampsia (Dalle Vedove et al., 2016; Santos et al., 2017; Sari et al., 2017). Given that few studies have investigated the physiological implications of sEH inhibition during pregnancy (Cizkova et al., 2016), future studies of sEH activity in pregnancy and reproduction are critical.
6. Considerations for future epidemiologic studies
As we have described, there is a growing body of evidence that prenatal oxylipin concentrations are associated with key pregnancy outcomes, as well as with prevalent environmental exposures. We would like to provide some considerations for future epidemiologic studies of oxylipins in pregnancy, regardless of whether the focus is on pregnancy outcomes or environmental exposures. Our suggestions primarily focus on the need for examining oxylipins across major biosynthetic pathways and the careful consideration for gestational timing that biological samples are collected.
6.1. Expanding from evaluating single markers to panels of oxylipins
Our review identified relatively few studies that simultaneously evaluated oxylipins from several biosynthetic pathways. However, the studies that did perform such analyses provided evidence that associations with health outcomes or chemical exposures differ across biosynthetic pathways (Martens 2017, Aung 2019, Borkowski 2020, Oliveira Perucci 2020, Welch 2020a, Aung 2021, Welch 2021). This strongly emphasizes the need for future studies to prioritize the targeted quantification of oxylipins across production pathways, including expanded measurement of enzymatic and nonenzymatic pathways. For example, we did not identify any studies that investigated oxylipins produced nonenzymatically from non-AA PUFAs, such as from DHA or EPA (Milne et al., 2015). This is a limitation in the field because these products may have promising biomarker potential (Galano et al., 2015).
Quantitatively targeting a large number of oxylipins simultaneously requires newer lipidomic technologies with high resolution, most of which use liquid chromatography with tandem mass spectrometry (LC-MS/MS) (Sorgi et al., 2018). The evolution of lipidomics with these new methods has enabled simultaneously quantifying oxylipins and other bioactive lipids across many biosynthetic pathways (Quehenberger and Dennis, 2011). Many of the studies we reviewed that analyzed a single or small number of oxylipin analytes utilized other methodological approaches such as immunoassays. The enzyme-linked immunosorbent assay (ELISA) is one most common immunoassays for quantifying oxylipins due to its relatively high cost-effectiveness, easy-to-use test kits, and good sensitivity for certain analytes (e.g., 8-isoprostane) (Liakh et al., 2020). However, ELISA methods are typically limited to measuring one analyte at a time and can have poor specificity (Il’yasova et al., 2004). Alternatively, LC-MS/MS methods provide substantially higher sensitivity and specificity and can simultaneously quantify a large number of oxylipins across all major biosynthetic pathways, but require highly technical purification steps and can still have issues separating similarly structured analytes (e.g., isomers) (Liakh et al., 2020). Targeted LC-MS/MS approaches are likely the best option for accurately quantifying a large number of oxylipin analytes in human samples, but untargeted approaches may become increasingly useful for identifying potentially important, yet unstudied, bioactive lipids in pregnancy (Liakh et al., 2020).
6.2. Biospecimen collection and evaluation
While expanding the list of oxylipin analytes to target is important, the biological matrix (e.g., serum, plasma, urine) used to target each biosynthetic pathway should always be carefully considered. For example, PUFA metabolism by COX enzymes may be more rapid and localized than other enzymes, so urine may provide a more accurate matrix than plasma for COX-produced oxylipins (Wu et al., 2010). However, plasma may be most representative of underlying inflammatory processes for oxylipins produced from most other enzymatic pathways (Dennis and Norris, 2015). Inconsistencies in published associations may stem from the fact that measures of oxylipins in maternal circulation (i.e., blood or urine) may not accurately reflect dysregulated production in specific tissues relevant to a particular condition (e.g., placenta versus maternal vasculature). While circulating oxylipin production may reflect causal inflammatory processes (Dennis and Norris, 2015) and are much more feasible than tissues in human studies, tissue-specific oxylipin production may be most relevant for understanding certain physiological processes. However, uterine tissues or fluids can be invasive or infeasible to collect in large populations, so future epidemiologic studies may benefit from alternative approaches. Exosomes have been suggested as a promising alternative to identifying links between systemic oxylipin levels and production in specific reproductive tissues (Mosaad et al., 2020). However, considerable work remains to enable exosomes and related complex molecules to be applicable to most epidemiologic study designs (Condrat et al., 2021).
Key methodological details related to biospecimen analysis also must be carefully considered when interpreting results. Most studies we examined measured free oxylipins (i.e., not covalently bound into phospholipids [non-esterified]); however, some studies measured total oxylipins (i.e., both free and esterified oxylipins). Free oxylipins are generally considered to be more bioactive (Dennis and Norris, 2015), but esterified oxylipins may have some relevant bioactivity (Spector, 2009). Regardless of portion (free or total), plasma is likely the best sample matrix to evaluate circulating oxylipins in blood (Rund et al., 2020). Serum was used in a number of studies, but serum can be problematic because of large increases in the ex vivo formation of oxylipins (e.g., COX-derived prostanoids, LOX-derived HETEs) during clotting reactions that typically occur at room-temperature (Rund et al., 2020). Urinary concentrations of oxylipins, particularly prostaglandins and isoprostanes, are correlated with plasma oxylipins and are often predictive of health outcomes (Milne et al., 2015). However, urinary oxylipins do need to be interpreted cautiously as concentrations may represent production in the liver or kidney rather than via systemic production processes (Milne et al., 2015; Mitchell et al., 2018).
Study design should also consider how natural and behavioral factors may influence oxylipin levels and potentially bias associations. For example, time of day of sample collection may influence oxylipin production based on natural diurnal variation (Colas et al., 2018) or due to recent dietary patterns (Ostermann et al., 2018; Gabbs et al., 2021). This variation in oxylipin levels could potentially confound associations with environmental exposures, as time of day of sample collection has also been associated with urinary chemical concentrations (Preau et al., 2010; Ferguson et al., 2014b; Philippat and Calafat, 2021). Although it may be infeasible to collect biological samples across participants at the same time of day, every effort should be made to at least record the time of day of sampling. This information can be valuable to perform key sensitivity analyses that address how time of day may be influencing associations. Similarly, future studies should also carefully consider whether to collect fasting or non-fasting samples and how this may influence associations with health outcomes or environmental exposures.
Free (non-esterified) oxylipins reflect the active signaling molecules and thus may be more physiologically-relevant than esterified forms for inflammatory or vasodilatory processes (Dennis and Norris, 2015). Fasting status can influence the levels of free and esterified PUFAs in circulation, but it remains unclear whether enzymatically-derived oxylipins in free or total form in circulation better represent the tissue-specific formation of oxylipins that may influence disease progression (Spector, 2009; Dennis and Norris, 2015). Recent meal intake can influence circulating levels of enzymatically-derived oxylipins (Gouveia-Figueira et al., 2015), while the nonenzymatically-derived isoprostanes are much less susceptible to postprandial changes (Gopaul et al., 2000; Kurti et al., 2017). Although there is less potential for dietary bias with isoprostanes derived from chemical oxidation, there are still important considerations regarding the sample matrix and laboratory analysis. In urine, isoprostanes can be directly excreted or metabolized to a variety of analytes (Milne et al., 2015). In plasma, isoprostanes are found in both free and esterified forms, but can have significant issues with artificial ex vivo formation (Milne et al., 2015). Although both urinary and plasma forms of isoprostanes can provide relevant insights into oxidative stress (Milne et al., 2015), it has been suggested that the best measurement of isoprostanes is to include total levels in plasma (free and esterified) and urine (free and metabolized) (Halliwell and Lee, 2010).
6.3. Selecting the gestational window for oxylipin measurement
Only a handful of studies used repeated sampling to investigate longitudinal changes in circulating oxylipins across pregnancy, as most used single spot sampling. Repeated sampling can provide insight into potential periods of susceptibility, but ultimately the choice of repeated versus spot sampling may be condition-specific. For example, our review showed that oxylipin production in mid to late pregnancy may be most relevant for preterm birth, thus indicating that sampling during these periods can provide relevant insights into potential mechanisms involving inflammation. However, it is possible that other conditions like preeclampsia and fetal growth restriction may be more likely to have differential oxylipin production in early pregnancy. An initial study with repeated plasma sampling has demonstrated that maternal oxylipin production may change in a non-linear pattern across pregnancy (Welch et al., 2020a). These results closely correspond to evidence from other maternal immunological processes, such as non-linear changes in the balance of T helper type (Th) 1 to Th2 response profiles (Mor et al., 2011). Thus, future studies should consider utilizing repeated sampling to account for this natural variation and better identify potential periods of susceptibility to dysregulated inflammation for the pregnancy outcome of interest.
Additionally, it is likely that periods outside of pregnancy have important implications for oxylipin production during pregnancy. Our review was focused on studies investigating oxylipin levels during pregnancy or at delivery, but evaluating oxylipin production during preconception and postpartum periods may provide valuable insights into health processes. For example, evidence suggests that preconception inflammation and cardiometabolic factors may be important factors for maternal and fetal pregnancy outcomes (Magnussen et al., 2007; Quesada et al., 2020). Thus, additional evidence is needed to understand how oxylipin production naturally fluctuates from preconception to postpartum in order to establish potential links to adverse outcomes.
6.4. Moving towards understanding causality
The causal role played by oxylipins in disease progression is still uncertain. In this review, we have evaluated evidence for the hypothesis that circulating oxylipins represent an important indicator of underlying tissue production preceding or exacerbating disease. However, there is still uncertainty as to whether oxylipin production may cause or change as a result of disease progression, as progression may lead to compensatory alterations in oxylipin formation (Dennis and Norris, 2015). For example, Oni-Orisan et al. (2016) found that plasma EETs were positively associated with the progression of certain subtypes of coronary artery disease (CAD). This finding would seem paradoxical, as EETs have anti-inflammatory, anti-atherosclerotic, and vasodilatory properties. However, increasing EET concentrations could be a compensatory protection process that helps mitigate disease severity. The study by Oni-Orisan et al. (2016) observed that patients with the highest plasma EET levels had the best long-term outcomes, including higher EETs among patients with nonobstructive rather than obstructive CAD (Oni-Orisan et al., 2016). Similar to compensatory effects, the direction of causality between oxylipins and diseases may be difficult to ascertain. Oxylipins such as isoprostanes can be both biomarkers of disease such as poor placental perfusion, but also act to reduce placental blood flow through activation of vasoconstrictive thromboxane receptors (Milne et al., 2015). Along with experimental studies, well-designed prospective epidemiologic studies that perform longitudinal biospecimen collection can help ascertain causal roles of potential compensatory effects and directionality of associations.
7. Concluding remarks
The epidemiologic evidence accumulated over recent years has provided evidence that inflammation and oxidative stress are critical factors involved with both healthy and pathological pregnancy. Exposure to environmental pollutants during pregnancy may adversely impact pregnancy by acting upon these inflammatory and oxidative stress pathways. The bioactive effects of oxylipins and their utility as biomarkers of inflammation and oxidative stress has made them exceptional candidates for investigating these links and provide a potential avenue for future therapeutic and diagnostic approaches to improving pregnancy outcomes. Further research is needed to establish which biosynthetic pathways of oxylipin production are most relevant for distinct health processes in pregnancy, as well as how specific environmental exposures may differentially impact these pathways. Improving the standardization of study design across pregnancy studies using the recommended approaches can help improve the impact future research has on maternal-fetal health.
Acknowledgements
This work was supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences.
Acronyms
- 8-isoprostane
8-iso-prostaglandin-F2α
- AA
arachidonic acid
- COX
cyclooxygenase
- CYP
cytochrome P450
- DHA
docosahexaenoic acid
- DHET
dihydroxy-eicosatrienoic acid
- DiHDPA
dihydroxy-docosapentaenoic acid
- DiHETE
dihydroxy-eicosatetraenoic acid
- DiHOME
dihydroxy-octadecenoic acid
- EET
epoxyeicosatrienoic acid
- EPA
eicosapentaenoic acid
- EpDPE
epoxy-docosapentaenoic acid
- EpETE
epoxy-eicosatetraenoic acid
- EpOME
epoxy-octadecenoic acid
- HETE
hydroxyeicosatetraenoic acid
- HODE
hydroxy-octadecadienoic acid
- LA
linoleic acid
- LGA
large for gestational age
- LOX
lipoxygenase
- LT
leukotriene
- LX
lipoxin
- PG
prostaglandin
- PGI
prostacyclin
- RBC
red blood cell
- SGA
small for gestational age
- TX
thromboxane
- IUGR
intrauterine growth restriction
- SGA
small for gestational age
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest Statement
The authors declare that there are no conflicts of interest.
References
- Abalos E, Cuesta C, Grosso AL, Chou D and Say L (2013). “Global and regional estimates of preeclampsia and eclampsia: a systematic review.” Eur J Obstet Gynecol Reprod Biol 170(1): 1–7. [DOI] [PubMed] [Google Scholar]
- Alfirevic Z, Keeney E, Dowswell T, Welton NJ, Dias S, Jones LV, Navaratnam K and Caldwell DM (2015). “Labour induction with prostaglandins: a systematic review and network meta-analysis.” BMJ 350: h217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambroz A, Vlkova V, Rossner P Jr., Rossnerova A, Svecova V, Milcova A, Pulkrabova J, Hajslova J, Veleminsky M Jr., Solansky I and Sram RJ (2016). “Impact of air pollution on oxidative DNA damage and lipid peroxidation in mothers and their newborns.” Int J Hyg Environ Health 219(6): 545–556. [DOI] [PubMed] [Google Scholar]
- American College of Obstetricians and Gynecologists (ACOG) (2016). “Practice Bulletin No. 171: Management of Preterm Labor.” Obstet Gynecol 128(4): e155–164. [DOI] [PubMed] [Google Scholar]
- American College of Obstetricians and Gynecologists (ACOG) (2021). “Fetal Growth Restriction: ACOG Practice Bulletin, Number 227.” Obstet Gynecol 137(2): e16–e28. [DOI] [PubMed] [Google Scholar]
- American College of Obstetrics and Gynecology (ACOG) (2018). “ACOG Committee Opinion No. 743: Low-Dose Aspirin Use During Pregnancy.” Obstet Gynecol 132(1): e44–e52. [DOI] [PubMed] [Google Scholar]
- American College of Obstetrics and Gynecology (ACOG) (2020). “Gestational Hypertension and Preeclampsia: ACOG Practice Bulletin, Number 222.” Obstet Gynecol 135(6): e237–e260. [DOI] [PubMed] [Google Scholar]
- Ander SE, Diamond MS and Coyne CB (2019). “Immune responses at the maternal-fetal interface.” Sci Immunol 4(31). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arogbokun O, Rosen E, Keil AP, Milne GL, Barrett E, Nguyen R, Bush NR, Swan SH, Sathyanarayana S and Ferguson KK (2021). “Maternal Oxidative Stress Biomarkers in Pregnancy and Child Growth from Birth to Age 6.” J Clin Endocrinol Metab 106(5): 1427–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashrap P, Watkins DJ, Milne GL, Ferguson KK, Loch-Caruso R, Fernandez J, Rosario Z, Velez-Vega CM, Alshawabkeh A, Cordero JF and Meeker JD (2021). “Maternal Urinary Metal and Metalloid Concentrations in Association with Oxidative Stress Biomarkers.” Antioxidants (Basel) 10(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aung MT, Song Y, Ferguson KK, Cantonwine DE, Zeng L, McElrath TF, Pennathur S, Meeker JD and Mukherjee B (2020). “Application of an analytical framework for multivariate mediation analysis of environmental data.” Nat Commun 11(1): 5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aung MT, Yu Y, Ferguson KK, Cantonwine DE, Zeng L, McElrath TF, Pennathur S, Mukherjee B and Meeker JD (2019). “Prediction and associations of preterm birth and its subtypes with eicosanoid enzymatic pathways and inflammatory markers.” Sci Rep 9(1): 17049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aung MT, Yu Y, Ferguson KK, Cantonwine DE, Zeng L, McElrath TF, Pennathur S, Mukherjee B and Meeker JD (2021). “Cross-Sectional Estimation of Endogenous Biomarker Associations with Prenatal Phenols, Phthalates, Metals, and Polycyclic Aromatic Hydrocarbons in Single-Pollutant and Mixtures Analysis Approaches.” Environ Health Perspect 129(3): 37007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ (2006). “Adult consequences of fetal growth restriction.” Clin Obstet Gynecol 49(2): 270–283. [DOI] [PubMed] [Google Scholar]
- Bazavilvaso-Rodriguez MA, Hernandez-Valencia M, Santillan-Morelos JG, Galvan-Duarte RE, Campos-Leon S, Lemus-Rocha SR, Saucedo R and Zarate A (2011). “Oxidative stress changes in pregnant patients with and without severe preeclampsia.” Arch Med Res 42(3): 195–198. [DOI] [PubMed] [Google Scholar]
- Bellien J and Joannides R (2013). “Epoxyeicosatrienoic acid pathway in human health and diseases.” J Cardiovasc Pharmacol 61(3): 188–196. [DOI] [PubMed] [Google Scholar]
- Bilodeau JF, Qin Wei S, Larose J, Greffard K, Moisan V, Audibert F, Fraser WD and Julien P (2015). “Plasma F2-isoprostane class VI isomers at 12-18 weeks of pregnancy are associated with later occurrence of preeclampsia.” Free Radic Biol Med 85: 282–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blencowe H, Cousens S, Chou D, Oestergaard M, Say L, Moller AB, Kinney M, Lawn J and Born G Too Soon Preterm Birth Action (2013). “Born too soon: the global epidemiology of 15 million preterm births.” Reprod Health 10 Suppl 1: S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borkowski K, Newman JW, Aghaeepour N, Mayo JA, Blazenovic I, Fiehn O, Stevenson DK, Shaw GM and Carmichael SL (2020). “Mid-gestation serum lipidomic profile associations with spontaneous preterm birth are influenced by body mass index.” PLoS One 15(11): e0239115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodin P, Jojic V, Gao T, Bhattacharya S, Angel CJ, Furman D, Shen-Orr S, Dekker CL, Swan GE, Butte AJ, Maecker HT and Davis MM (2015). “Variation in the human immune system is largely driven by non-heritable influences.” Cell 160(1-2): 37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buczynski MW, Dumlao DS and Dennis EA (2009). “Thematic Review Series: Proteomics. An integrated omics analysis of eicosanoid biology.” J Lipid Res 50(6): 1015–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappelletti M, Della Bella S, Ferrazzi E, Mavilio D and Divanovic S (2016). “Inflammation and preterm birth.” J Leukoc Biol 99(1): 67–78. [DOI] [PubMed] [Google Scholar]
- Carlin DJ, Rider CV, Woychik R and Birnbaum LS (2013). “Unraveling the health effects of environmental mixtures: an NIEHS priority.” Environ Health Perspect 121(1): A6–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson SE, Colombo J, Gajewski BJ, Gustafson KM, Mundy D, Yeast J, Georgieff MK, Markley LA, Kerling EH and Shaddy DJ (2013). “DHA supplementation and pregnancy outcomes.” Am J Clin Nutr 97(4): 808–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cathey AL, Eaton JL, Ashrap P, Watkins DJ, Rosario ZY, Velez Vega C, Alshawabkeh AN, Cordero JF, Mukherjee B and Meeker JD (2021). “Individual and joint effects of phthalate metabolites on biomarkers of oxidative stress among pregnant women in Puerto Rico.” Environ Int 154: 106565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell LC, Seed PT, Briley AL, Kelly FJ, Lee R, Hunt BJ, Parmar K, Bewley SJ, Shennan AH, Steer PJ and Poston L (1999). “Effect of antioxidants on the occurrence of pre-eclampsia in women at increased risk: a randomised trial.” Lancet 354(9181): 810–816. [DOI] [PubMed] [Google Scholar]
- Cizkova K, Rajdova A and Ehrmann J (2016). “Soluble Epoxide Hydrolase as a Potential Key Factor for Human Prenatal Development.” Cells Tissues Organs 201(4): 277–286. [DOI] [PubMed] [Google Scholar]
- Cohen AJ, Brauer M, Burnett R, Anderson HR, Frostad J, Estep K, Balakrishnan K, Brunekreef B, Dandona L, Dandona R, Feigin V, Freedman G, Hubbell B, Jobling A, Kan H, Knibbs L, Liu Y, Martin R, Morawska L, Pope CA 3rd, Shin H, Straif K, Shaddick G, Thomas M, van Dingenen R, van Donkelaar A, Vos T, Murray CJL and Forouzanfar MH (2017). “Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study 2015.” Lancet 389(10082): 1907–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colas RA, Souza PR, Walker ME, Burton M, Zaslona Z, Curtis AM, Marques RM and Dalli J (2018). “Impaired Production and Diurnal Regulation of Vascular RvDn-3 DPA Increase Systemic Inflammation and Cardiovascular Disease.” Circ Res 122(6): 855–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condrat CE, Varlas VN, Duica F, Antoniadis P, Danila CA, Cretoiu D, Suciu N, Cretoiu SM and Voinea SC (2021). “Pregnancy-Related Extracellular Vesicles Revisited.” Int J Mol Sci 22(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalle Vedove F, Fava C, Jiang H, Zanconato G, Quilley J, Brunelli M, Guglielmi V, Vattemi G and Minuz P (2016). “Increased epoxyeicosatrienoic acids and reduced soluble epoxide hydrolase expression in the preeclamptic placenta.” J Hypertens 34(7): 1364–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dashner-Titus EJ, Hoover J, Li L, Lee JH, Du R, Liu KJ, Traber MG, Ho E, Lewis J and Hudson LG (2018). “Metal exposure and oxidative stress markers in pregnant Navajo Birth Cohort Study participants.” Free Radic Biol Med 124: 484–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Sousa Rocha V, Della Rosa FB, Ruano R, Zugaib M and Colli C (2015). “Association between magnesium status, oxidative stress and inflammation in preeclampsia: A case-control study.” Clin Nutr 34(6): 1166–1171. [DOI] [PubMed] [Google Scholar]
- Dennis EA and Norris PC (2015). “Eicosanoid storm in infection and inflammation.” Nat Rev Immunol 15(8): 511–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietert RR (2009). “Developmental immunotoxicology: focus on health risks.” Chem Res Toxicol 22(1): 17–23. [DOI] [PubMed] [Google Scholar]
- Djuricic I and Calder PC (2021). “Beneficial Outcomes of Omega-6 and Omega-3 Polyunsaturated Fatty Acids on Human Health: An Update for 2021.” Nutrients 13(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong W and Yin L (2014). “Expression of lipoxin A4, TNFalpha and IL-1beta in maternal peripheral blood, umbilical cord blood and placenta, and their significance in pre-eclampsia.” Hypertens Pregnancy 33(4): 449–456. [DOI] [PubMed] [Google Scholar]
- Duhig K, Chappell LC and Shennan AH (2016). “Oxidative stress in pregnancy and reproduction.” Obstet Med 9(3): 113–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edin ML, Duval C, Zhang G and Zeldin DC (2020). “Role of linoleic acid-derived oxylipins in cancer.” Cancer Metastasis Rev 39(3): 581–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eick SM, Ferguson KK, Milne GL, Rios-McConnell R, Velez-Vega C, Rosario Z, Alshawabkeh A, Cordero JF and Meeker JD (2020). “Repeated measures of urinary oxidative stress biomarkers and preterm birth in Puerto Rico.” Free Radic Biol Med 146: 299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott E, Hanson CK, Anderson-Berry AL and Nordgren TM (2017). “The role of specialized pro-resolving mediators in maternal-fetal health.” Prostaglandins Leukot Essent Fatty Acids 126: 98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst GD, de Jonge LL, Hofman A, Lindemans J, Russcher H, Steegers EA and Jaddoe VW (2011). “C-reactive protein levels in early pregnancy, fetal growth patterns, and the risk for neonatal complications: the Generation R Study.” Am J Obstet Gynecol 205(2): 132 e131–112. [DOI] [PubMed] [Google Scholar]
- Esser-von Bieren J (2017). “Immune-regulation and -functions of eicosanoid lipid mediators.” Biol Chem 398(11): 1177–1191. [DOI] [PubMed] [Google Scholar]
- European Food Safety Authority Panel on Dietetic Products, N. a. A. E. (2014). “Scientific Opinion on the essential composition of infant and follow-on formulae.” EFSA Journal 12(7). [Google Scholar]
- Ferguson KK, Cantonwine DE, McElrath TF, Mukherjee B and Meeker JD (2016). “Repeated measures analysis of associations between urinary bisphenol-A concentrations and biomarkers of inflammation and oxidative stress in pregnancy.” Reprod Toxicol 66: 93–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KK, Cantonwine DE, Rivera-Gonzalez LO, Loch-Caruso R, Mukherjee B, Anzalota Del Toro LV, Jimenez-Velez B, Calafat AM, Ye X, Alshawabkeh AN, Cordero JF and Meeker JD (2014a). “Urinary phthalate metabolite associations with biomarkers of inflammation and oxidative stress across pregnancy in Puerto Rico.” Environ Sci Technol 48(12): 7018–7025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KK, Chen YH, VanderWeele TJ, McElrath TF, Meeker JD and Mukherjee B (2017a). “Mediation of the Relationship between Maternal Phthalate Exposure and Preterm Birth by Oxidative Stress with Repeated Measurements across Pregnancy.” Environ Health Perspect 125(3): 488–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KK and Chin HB (2017). “Environmental chemicals and preterm birth: Biological mechanisms and the state of the science.” Curr Epidemiol Rep 4(1): 56–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KK, Kamai EM, Cantonwine DE, Mukherjee B, Meeker JD and McElrath TF (2018). “Associations between repeated ultrasound measures of fetal growth and biomarkers of maternal oxidative stress and inflammation in pregnancy.” Am J Reprod Immunol 80(4): e13017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KK, Lan Z, Yu Y, Mukherjee B, McElrath TF and Meeker JD (2019). “Urinary concentrations of phenols in association with biomarkers of oxidative stress in pregnancy: Assessment of effects independent of phthalates.” Environ Int 131: 104903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KK, McElrath TF, Chen YH, Loch-Caruso R, Mukherjee B and Meeker JD (2015). “Repeated measures of urinary oxidative stress biomarkers during pregnancy and preterm birth.” Am J Obstet Gynecol 212(2): 208 e201–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KK, McElrath TF, Ko YA, Mukherjee B and Meeker JD (2014b). “Variability in urinary phthalate metabolite levels across pregnancy and sensitive windows of exposure for the risk of preterm birth.” Environ Int 70: 118–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KK, McElrath TF, Pace GG, Weller D, Zeng L, Pennathur S, Cantonwine DE and Meeker JD (2017b). “Urinary Polycyclic Aromatic Hydrocarbon Metabolite Associations with Biomarkers of Inflammation, Angiogenesis, and Oxidative Stress in Pregnant Women.” Environ Sci Technol 51(8): 4652–4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KK, Meeker JD, McElrath TF, Mukherjee B and Cantonwine DE (2017c). “Repeated measures of inflammation and oxidative stress biomarkers in preeclamptic and normotensive pregnancies.” Am J Obstet Gynecol 216(5): 527 e521–527 e529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, Franceschi C, Ferrucci L, Gilroy DW, Fasano A, Miller GW, Miller AH, Mantovani A, Weyand CM, Barzilai N, Goronzy JJ, Rando TA, Effros RB, Lucia A, Kleinstreuer N and Slavich GM (2019). “Chronic inflammation in the etiology of disease across the life span.” Nat Med 25(12): 1822–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbs M, Leng S, Devassy JG, Monirujjaman M and Aukema HM (2015). “Advances in Our Understanding of Oxylipins Derived from Dietary PUFAs.” Adv Nutr 6(5): 513–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbs M, Zahradka P, Taylor CG and Aukema HM (2021). “Time Course and Sex Effects of alpha-Linolenic Acid-Rich and DHA-Rich Supplements on Human Plasma Oxylipins: A Randomized Double-Blind Crossover Trial.” J Nutr 151(3): 513–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galano JM, Lee YY, Durand T and Lee JC (2015). “Special Issue on “Analytical Methods for Oxidized Biomolecules and Antioxidants” The use of isoprostanoids as biomarkers of oxidative damage, and their role in human dietary intervention studies.” Free Radic Res 49(5): 583–598. [DOI] [PubMed] [Google Scholar]
- Genc H, Uzun H, Benian A, Simsek G, Gelisgen R, Madazli R and Guralp O (2011). “Evaluation of oxidative stress markers in first trimester for assessment of preeclampsia risk.” Arch Gynecol Obstet 284(6): 1367–1373. [DOI] [PubMed] [Google Scholar]
- Gopaul NK, Zacharowski K, Halliwell B and Anggard EE (2000). “Evaluation of the postprandial effects of a fast-food meal on human plasma F(2)-isoprostane levels.” Free Radic Biol Med 28(5): 806–814. [DOI] [PubMed] [Google Scholar]
- Gouveia-Figueira S, Spath J, Zivkovic AM and Nording ML (2015). “Profiling the Oxylipin and Endocannabinoid Metabolome by UPLC-ESI-MS/MS in Human Plasma to Monitor Postprandial Inflammation.” PLoS One 10(7): e0132042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant MJ and Booth A (2009). “A typology of reviews: an analysis of 14 review types and associated methodologies.” Health Info Libr J 26(2): 91–108. [DOI] [PubMed] [Google Scholar]
- Halliwell B and Lee CY (2010). “Using isoprostanes as biomarkers of oxidative stress: some rarely considered issues.” Antioxid Redox Signal 13(2): 145–156. [DOI] [PubMed] [Google Scholar]
- Hansen C, Neller A, Williams G and Simpson R (2006). “Maternal exposure to low levels of ambient air pollution and preterm birth in Brisbane, Australia.” BJOG 113(8): 935–941. [DOI] [PubMed] [Google Scholar]
- Hantsoo L, Kornfield S, Anguera MC and Epperson CN (2019). “Inflammation: A Proposed Intermediary Between Maternal Stress and Offspring Neuropsychiatric Risk.” Biol Psychiatry 85(2): 97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon AC, Cornelius DC, Amaral LM, Faulkner JL, Cunningham MW Jr., Wallace K and LaMarca B (2016). “The role of inflammation in the pathology of preeclampsia.” Clin Sci (Lond) 130(6): 409–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herse F, Lamarca B, Hubel CA, Kaartokallio T, Lokki AI, Ekholm E, Laivuori H, Gauster M, Huppertz B, Sugulle M, Ryan MJ, Novotny S, Brewer J, Park JK, Kacik M, Hoyer J, Verlohren S, Wallukat G, Rothe M, Luft FC, Muller DN, Schunck WH, Staff AC and Dechend R (2012). “Cytochrome P450 subfamily 2J polypeptide 2 expression and circulating epoxyeicosatrienoic metabolites in preeclampsia.” Circulation 126(25): 2990–2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland N, Huen K, Tran V, Street K, Nguyen B, Bradman A and Eskenazi B (2016). “Urinary Phthalate Metabolites and Biomarkers of Oxidative Stress in a Mexican-American Cohort: Variability in Early and Late Pregnancy.” Toxics 4(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong JS, Romero R, Lee DC, Than NG, Yeo L, Chaemsaithong P, Ahn S, Kim JS, Kim CJ and Kim YM (2016). “Umbilical cord prostaglandins in term and preterm parturition.” J Matern Fetal Neonatal Med 29(4): 523–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh TT, Chen SF, Lo LM, Li MJ, Yeh YL and Hung TH (2012). “The association between maternal oxidative stress at mid-gestation and subsequent pregnancy complications.” Reprod Sci 19(5): 505–512. [DOI] [PubMed] [Google Scholar]
- Huang YF, Wang PW, Huang LW, Lai CH, Yang W, Wu KY, Lu CA, Chen HC and Chen ML (2017). “Prenatal Nonylphenol and Bisphenol A Exposures and Inflammation Are Determinants of Oxidative/Nitrative Stress: A Taiwanese Cohort Study.” Environ Sci Technol 51 (11): 6422–6429. [DOI] [PubMed] [Google Scholar]
- Il’yasova D, Morrow JD, Ivanova A and Wagenknecht LE (2004). “Epidemiological marker for oxidant status: comparison of the ELISA and the gas chromatography/mass spectrometry assay for urine 2,3-dinor-5,6-dihydro-15-F2t-isoprostane.” Ann Epidemiol 14(10): 793–797. [DOI] [PubMed] [Google Scholar]
- Ilhan N, Celik E and Kumbak B (2015). “Maternal plasma levels of interleukin-6, C-reactive protein, vitamins C, E and A, 8-isoprostane and oxidative status in women with preterm premature rupture of membranes.” J Matern Fetal Neonatal Med 28(3): 316–319. [DOI] [PubMed] [Google Scholar]
- Imig JD and Hammock BD (2009). “Soluble epoxide hydrolase as a therapeutic target for cardiovascular diseases.” Nat Rev Drug Discov 8(10): 794–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingle ME, Watkins D, Rosario Z, VelezVega CM, Calafat AM, Ospina M, Ferguson KK, Cordero JF, Alshawabkeh A and Meeker JD (2020). “An exploratory analysis of urinary organophosphate ester metabolites and oxidative stress among pregnant women in Puerto Rico.” Sci Total Environ 703: 134798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, McGiff JC, Fava C, Amen G, Nesta E, Zanconato G, Quilley J and Minuz P (2013). “Maternal and fetal epoxyeicosatrienoic acids in normotensive and preeclamptic pregnancies.” Am J Hypertens 26(2): 271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn LG and Trasande L (2018). “Environmental Toxicant Exposure and Hypertensive Disorders of Pregnancy: Recent Findings.” Curr Hypertens Rep 20(10): 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamai EM, McElrath TF and Ferguson KK (2019). “Fetal growth in environmental epidemiology: mechanisms, limitations, and a review of associations with biomarkers of non-persistent chemical exposures during pregnancy.” Environ Health 18(1): 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SS, Meeker JD, Keil AP, Aung MT, Bommarito PA, Cantonwine DE, McElrath TF and Ferguson KK (2019). “Exposure to 17 trace metals in pregnancy and associations with urinary oxidative stress biomarkers.” Environ Res 179(Pt B): 108854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuc S, Koster MP, Pennings JL, Hankemeier T, Berger R, Harms AC, Dane AD, Schielen PC, Visser GH and Vreeken RJ (2014). “Metabolomics profiling for identification of novel potential markers in early prediction of preeclampsia.” PLoS One 9(5): e98540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumarathasan P, Vincent R, Bielecki A, Blais E, Blank K, Das D, Karthikeyan S, Cakmak S, Fisher M, Arbuckle T and Fraser W (2016). “Infant birth weight and third trimester maternal plasma markers of vascular integrity: the MIREC study.” Biomarkers 21(3): 257–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurti SP, Emerson SR, Rosenkranz SK, Teeman CS, Emerson EM, Cull BJ, Smith JR and Harms CA (2017). “Post-prandial systemic 8-isoprostane increases after consumption of moderate and high-fat meals in insufficiently active males.” Nutr Res 39: 61–68. [DOI] [PubMed] [Google Scholar]
- Larque E, Gil-Sanchez A, Prieto-Sanchez MT and Koletzko B (2012). “Omega 3 fatty acids, gestation and pregnancy outcomes.” Br J Nutr 107 Suppl 2: S77–84. [DOI] [PubMed] [Google Scholar]
- Lee SE, Romero R, Park IS, Seong HS, Park CW and Yoon BH (2008). “Amniotic fluid prostaglandin concentrations increase before the onset of spontaneous labor at term.” J Matern Fetal Neonatal Med 21 (2): 89–94. [DOI] [PubMed] [Google Scholar]
- Lewis DF, Canzoneri BJ, Gu Y, Zhao S and Wang Y (2010). “Maternal levels of prostacyclin, thromboxane, ICAM, and VCAM in normal and preeclamptic pregnancies.” Am J Reprod Immunol 64(6): 376–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Bradbury JA, Edin ML, Graves JP, Gruzdev A, Cheng J, Hoopes SL, DeGraff LM, Fessler MB, Garantziotis S, Schurman SH and Zeldin DC (2021). “sEH promotes macrophage phagocytosis and lung clearance of Streptococcus pneumoniae.” J Clin Invest 131(22). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Navab K, Hough G, Daher N, Zhang M, Mittelstein D, Lee K, Pakbin P, Saffari A, Bhetraratana M, Sulaiman D, Beebe T, Wu L, Jen N, Wine E, Tseng CH, Araujo JA, Fogelman A, Sioutas C, Navab M and Hsiai TK (2015). “Effect of exposure to atmospheric ultrafine particles on production of free fatty acids and lipid metabolites in the mouse small intestine.” Environ Health Perspect 123(1): 34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liakh I, Pakiet A, Sledzinski T and Mika A (2020). “Methods of the Analysis of Oxylipins in Biological Samples.” Molecules 25(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstrom E, Persson LA, Raqib R, El Arifeen S, Basu S and Ekstrom EC (2012). “Associations between oxidative parameters in pregnancy and birth anthropometry in a cohort of women and children in rural Bangladesh: the MINIMat-cohort.” Free Radic Res 46(3): 253–264. [DOI] [PubMed] [Google Scholar]
- Lipa M, Bomba-Opon D, Lipa J, Bartnik P, Bartoszewicz Z and Wielgos M (2017). “Lipoxin A4 (LXA4) as a potential first trimester biochemical marker of intrauterine growth disorders.” J Matern Fetal Neonatal Med 30(20): 2495–2497. [DOI] [PubMed] [Google Scholar]
- Liu S, Krewski D, Shi Y, Chen Y and Burnett RT (2007). “Association between maternal exposure to ambient air pollutants during pregnancy and fetal growth restriction.” J Expo Sci Environ Epidemiol 17(5): 426–432. [DOI] [PubMed] [Google Scholar]
- Longini M, Perrone S, Vezzosi P, Marzocchi B, Kenanidis A, Centini G, Rosignoli L and Buonocore G (2007). “Association between oxidative stress in pregnancy and preterm premature rupture of membranes.” Clin Biochem 40(11): 793–797. [DOI] [PubMed] [Google Scholar]
- Maciel-Ruiz JA, Lopez-Rivera C, Robles-Morales R, Veloz-Martinez MG, Lopez-Arellano R, Rodriguez-Patino G, Petrosyan P, Govezensky T, Salazar AM, Ostrosky-Wegman P, Montero-Montoya R and Gonsebatt ME (2019). “Prenatal exposure to particulate matter and ozone: Bulky DNA adducts, plasma isoprostanes, allele risk variants, and neonate susceptibility in the Mexico City Metropolitan Area.” Environ Mol Mutagen 60(5): 428–442. [DOI] [PubMed] [Google Scholar]
- Magnussen EB, Vatten LJ, Lund-Nilsen TI, Salvesen KA, Davey Smith G and Romundstad PR (2007). “Prepregnancy cardiovascular risk factors as predictors of pre-eclampsia: population based cohort study.” BMJ 335(7627): 978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandy G, Weisman L and Kim M. (2019). “Infants with fetal (intrauterine) growth restriction.” Retrieved 12/19/2019, from https://www.uptodate.com/contents/infants-with-fetal-intrauterine-growth-restriction.
- Martens DS, Gouveia S, Madhloum N, Janssen BG, Plusquin M, Vanpoucke C, Lefebvre W, Forsberg B, Nording M and Nawrot TS (2017). “Neonatal Cord Blood Oxylipins and Exposure to Particulate Matter in the Early-Life Environment: An ENVIRONAGE Birth Cohort Study.” Environ Health Perspect 125(4): 691–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick MC (1985). “The contribution of low birth weight to infant mortality and childhood morbidity.” N Engl J Med 312(2): 82–90. [DOI] [PubMed] [Google Scholar]
- McElrath TF, Hecht JL, Dammann O, Boggess K, Onderdonk A, Markenson G, Harper M, Delpapa E, Allred EN, Leviton A and Investigators ES (2008). “Pregnancy disorders that lead to delivery before the 28th week of gestation: an epidemiologic approach to classification.” Am J Epidemiol 168(9): 980–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestan K, Matoba N, Arguelles L, Harvey C, Ernst LM, Farrow K and Wang X (2012). “Cord blood 8-isoprostane in the preterm infant.” Early Hum Dev 88(8): 683–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne GL, Dai Q and Roberts LJ, 2nd (2015). “The isoprostanes--25 years later.” Biochim Biophys Acta 1851(4): 433–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JA, Knowles RB, Kirkby NS, Reed DM, Edin ML, White WE, Chan MV, Longhurst H, Yaqoob MM, Milne GL, Zeldin DC and Warner TD (2018). “Kidney Transplantation in a Patient Lacking Cytosolic Phospholipase A2 Proves Renal Origins of Urinary PGI-M and TX-M.” Circ Res 122(4): 555–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell MD, Rice GE, Vaswani K, Kvaskoff D and Peiris HN (2016). “Differential Regulation of Eicosanoid and Endocannabinoid Production by Inflammatory Mediators in Human Choriodecidua.” PLoS One 11(2): e0148306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mor G, Cardenas I, Abrahams V and Guller S (2011). “Inflammation and pregnancy: the role of the immune system at the implantation site.” Ann N Y Acad Sci 1221: 80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosaad E, Peiris HN, Holland O, Morean Garcia I and Mitchell MD (2020). “The Role(s) of Eicosanoids and Exosomes in Human Parturition.” Front Physiol 11: 594313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa M, Garg N, Banerjee BD, Sharma T, Tyagi V, Dar SA, Guleria K, Ahmad RS, Vaid N and Tripathi AK (2015). “Inflammatory-mediated pathway in association with organochlorine pesticides levels in the etiology of idiopathic preterm birth.” Reprod Toxicol 57: 111–120. [DOI] [PubMed] [Google Scholar]
- Negro S, Boutsikou T, Briana DD, Tataranno ML, Longini M, Proietti F, Bazzini F, Dani C, Malamitsi-Puchner A, Buonocore G and Perrone S (2017). “Maternal obesity and perinatal oxidative stress: the strength of the association.” J Biol Regul Homeost Agents 31(1): 221–227. [PubMed] [Google Scholar]
- Norwood S, Liao J, Hammock BD and Yang GY (2010). “Epoxyeicosatrienoic acids and soluble epoxide hydrolase: potential therapeutic targets for inflammation and its induced carcinogenesis.” Am J Transl Res 2(4): 447–457. [PMC free article] [PubMed] [Google Scholar]
- Olson DM and Ammann C (2007). “Role of the prostaglandins in labour and prostaglandin receptor inhibitors in the prevention of preterm labour.” Front Biosci 12: 1329–1343. [DOI] [PubMed] [Google Scholar]
- Oni-Orisan A, Edin ML, Lee JA, Wells MA, Christensen ES, Vendrov KC, Lih FB, Tomer KB, Bai X, Taylor JM, Stouffer GA, Zeldin DC and Lee CR (2016). “Cytochrome P450-derived epoxyeicosatrienoic acids and coronary artery disease in humans: a targeted metabolomics study.” J Lipid Res 57(1): 109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppedisano F, Macri R, Gliozzi M, Musolino V, Carresi C, Maiuolo J, Bosco F, Nucera S, Caterina Zito M, Guarnieri L, Scarano F, Nicita C, Coppoletta AR, Ruga S, Scicchitano M, Mollace R, Palma E and Mollace V (2020). “The Anti-Inflammatory and Antioxidant Properties of n-3 PUFAs: Their Role in Cardiovascular Protection.” Biomedicines 8(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oral E, Cagdas A, Gezer A, Kaleli S, Aydinli K and Ocer F (2001). “Perinatal and maternal outcomes of fetal macrosomia.” Eur J Obstet Gynecol Reprod Biol 99(2): 167–171. [DOI] [PubMed] [Google Scholar]
- Ostermann AI, Greupner T, Kutzner L, Hartung NM, Hahn A, Schuchardt JP and Schebb NH (2018). “Intra-individual variance of the human plasma oxylipin pattern: low inter-day variability in fasting blood samples versus high variability during the day.” Analytical Methods 10(40): 4935–4944. [Google Scholar]
- Panigrahy D, Gilligan MM, Serhan CN and Kashfi K (2021). “Resolution of inflammation: An organizing principle in biology and medicine.” Pharmacol Ther 227: 107879. [DOI] [PubMed] [Google Scholar]
- Park JY, Romero R, Lee J, Chaemsaithong P, Chaiyasit N and Yoon BH (2016). “An elevated amniotic fluid prostaglandin F2alpha concentration is associated with intra-amniotic inflammation/infection, and clinical and histologic chorioamnionitis, as well as impending preterm delivery in patients with preterm labor and intact membranes.” J Matern Fetal Neonatal Med 29(16): 2563–2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patra RC, Rautray AK and Swarup D (2011). “Oxidative stress in lead and cadmium toxicity and its amelioration.” Vet Med Int 2011: 457327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson T, Zhang J, Arya P, Warren AY, Ortori C, Fakis A, Khan RN and Barrett DA (2010). “Measurement of vasoactive metabolites (hydroxyeicosatetraenoic and epoxyeicosatrienoic acids) in uterine tissues of normal and compromised human pregnancy.” J Hypertens 28(12): 2429–2437. [DOI] [PubMed] [Google Scholar]
- Perucci LO, de Castro Pinto KM, da Silva SPG, Lage EM, Teixeira PG, Barbosa AS, Alpoim PN, de Sousa LP, Talvani A and Dusse LMS (2021). “Longitudinal assessment of leukotriene B4, lipoxin A4, and resolvin D1 plasma levels in pregnant women with risk factors for preeclampsia.” Clin Biochem 98: 24–28. [DOI] [PubMed] [Google Scholar]
- Perucci LO, Pereira Santos TA, Campi Santos P, Ribeiro Teixeira LC, Nessralla Alpoim P, Braga Gomes K, Pires Sousa L, Sant’Ana Dusse LM and Talvani A (2020). “Pre-eclampsia is associated with reduced resolvin D1 and maresin 1 to leukotriene B4 ratios in the plasma.” Am J Reprod Immunol 83(2): e13206. [DOI] [PubMed] [Google Scholar]
- Perucci LO, Santos PC, Ribeiro LS, Souza DG, Gomes KB, Dusse LM and Sousa LP (2016). “Lipoxin A4 Is Increased in the Plasma of Preeclamptic Women.” Am J Hypertens 29(10): 1179–1185. [DOI] [PubMed] [Google Scholar]
- Philippat C and Calafat AM (2021). “Comparison of strategies to efficiently combine repeated urine samples in biomarker-based studies.” Environ Res 192: 110275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plenty NL, Faulkner JL, Cotton J, Spencer SK, Wallace K, LaMarca B and Murphy SR (2018). “Arachidonic acid metabolites of CYP4A and CYP4F are altered in women with preeclampsia.” Prostaglandins Other Lipid Mediat 136: 15–22. [DOI] [PubMed] [Google Scholar]
- Poston L, Briley AL, Seed PT, Kelly FJ, Shennan AH and Vitamins C in Pre-eclampsia Trial (2006). “Vitamin C and vitamin E in pregnant women at risk for pre-eclampsia (VIP trial): randomised placebo-controlled trial.” Lancet 367(9517): 1145–1154. [DOI] [PubMed] [Google Scholar]
- Preau JL Jr., Wong LY, Silva MJ, Needham LL and Calafat AM (2010). “Variability over 1 week in the urinary concentrations of metabolites of diethyl phthalate and di(2-ethylhexyl) phthalate among eight adults: an observational study.” Environ Health Perspect 118(12): 1748–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quehenberger O and Dennis EA (2011). “The human plasma lipidome.” N Engl J Med 365(19): 1812–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesada O, Shufelt C and Bairey Merz CN (2020). “Can We Improve Cardiovascular Disease for Women Using Data Under Our Noses?: A Need for Changes in Policy and Focus.” JAMA Cardiol 5(12): 1398–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsden CE, Makrides M, Yuan ZX, Horowitz MS, Zamora D, Yelland LN, Best K, Jensen J, Taha AY and Gibson RA (2020). “Plasma oxylipins and unesterified precursor fatty acids are altered by DHA supplementation in pregnancy: Can they help predict risk of preterm birth?” Prostaglandins Leukot Essent Fatty Acids 153: 102041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehman K, Fatima F, Waheed I and Akash MSH (2018). “Prevalence of exposure of heavy metals and their impact on health consequences.” J Cell Biochem 119(1): 157–184. [DOI] [PubMed] [Google Scholar]
- Roberge S, Villa P, Nicolaides K, Giguere Y, Vainio M, Bakthi A, Ebrashy A and Bujold E (2012). “Early administration of low-dose aspirin for the prevention of preterm and term preeclampsia: a systematic review and meta-analysis.” Fetal Diagn Ther 31(3): 141–146. [DOI] [PubMed] [Google Scholar]
- Roberts JM, Myatt L, Spong CY, Thom EA, Hauth JC, Leveno KJ, Pearson GD, Wapner RJ, Varner MW, Thorp JM Jr., Mercer BM, Peaceman AM, Ramin SM, Carpenter MW, Samuels P, Sciscione A, Harper M, Smith WJ, Saade G, Sorokin Y, Anderson GB, Eunice H Kennedy Shriver National Institute of Child and N. Human Development Maternal-Fetal Medicine Units (2010). “Vitamins C and E to prevent complications of pregnancy-associated hypertension.” N Engl J Med 362(14): 1282–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DP and Klein SL (2012). “Pregnancy and pregnancy-associated hormones alter immune responses and disease pathogenesis.” Horm Behav 62(3): 263–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roland L, Gagne A, Belanger MC, Boutet M, Berthiaume L, Fraser WD, Julien P and Bilodeau JF (2010). “Existence of compensatory defense mechanisms against oxidative stress and hypertension in preeclampsia.” Hypertens Pregnancy 29(1): 21–37. [DOI] [PubMed] [Google Scholar]
- Romero R, Gotsch F, Pineles B and Kusanovic JP (2007). “Inflammation in pregnancy: its roles in reproductive physiology, obstetrical complications, and fetal injury.” Nutr Rev 65(12 Pt 2): S194–202. [DOI] [PubMed] [Google Scholar]
- Rosen EM, Munoz MI, McElrath T, Cantonwine DE and Ferguson KK (2018). “Environmental contaminants and preeclampsia: a systematic literature review.” J Toxicol Environ Health B Crit Rev 21(5): 291–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen EM, van’t Erve TJ, Boss J, Sathyanarayana S, Barrett ES, Nguyen RHN, Bush NR, Milne GL, McElrath TF, Swan SH and Ferguson KK (2019). “Urinary oxidative stress biomarkers and accelerated time to spontaneous delivery.” Free Radic Biol Med 130: 419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rund KM, Nolte F, Doricic J, Greite R, Schott S, Lichtinghagen R, Gueler F and Schebb NH (2020). “Clinical blood sampling for oxylipin analysis - effect of storage and pneumatic tube transport of blood on free and total oxylipin profile in human plasma and serum.” Analyst 145(6): 2378–2388. [DOI] [PubMed] [Google Scholar]
- Saccone G, Saccone I and Berghella V (2016). “Omega-3 long-chain polyunsaturated fatty acids and fish oil supplementation during pregnancy: which evidence?” J Matern Fetal Neonatal Med 29(15): 2389–2397. [DOI] [PubMed] [Google Scholar]
- Santos JM, Park JA, Joiakim A, Putt DA, Taylor RN and Kim H (2017). “The role of soluble epoxide hydrolase in preeclampsia.” Med Hypotheses 108: 81–85. [DOI] [PubMed] [Google Scholar]
- Sari I, Pinarbasi H, Pinarbasi E and Yildiz C (2017). “Association between the soluble epoxide hydrolase gene and preeclampsia.” Hypertens Pregnancy 36(4): 315–325. [DOI] [PubMed] [Google Scholar]
- Schieber M and Chandel NS (2014). “ROS function in redox signaling and oxidative stress.” Curr Biol 24(10): R453–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN (2014). “Pro-resolving lipid mediators are leads for resolution physiology.” Nature 510(7503): 92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen HC and Hammock BD (2012). “Discovery of inhibitors of soluble epoxide hydrolase: a target with multiple potential therapeutic indications.” J Med Chem 55(5): 1789–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simopoulos AP (2016). “An Increase in the Omega-6/Omega-3 Fatty Acid Ratio Increases the Risk for Obesity.” Nutrients 8(3): 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorgi CA, Peti APF, Petta T, Meirelles AFG, Fontanari C, Moraes LAB and Faccioli LH (2018). “Comprehensive high-resolution multiple-reaction monitoring mass spectrometry for targeted eicosanoid assays.” Sci Data 5: 180167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector AA (2009). “Arachidonic acid cytochrome P450 epoxygenase pathway.” J Lipid Res 50 Suppl: S52–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector AA, Fang X, Snyder GD and Weintraub NL (2004). “Epoxyeicosatrienoic acids (EETs): metabolism and biochemical function.” Prog Lipid Res 43(1): 55–90. [DOI] [PubMed] [Google Scholar]
- Steegers EA, von Dadelszen P, Duvekot JJ and Pijnenborg R (2010). “Pre-eclampsia.” Lancet 376(9741): 631–644. [DOI] [PubMed] [Google Scholar]
- Strauss JF, Barbieri RL and Yen SSC (2018). Chapter 4 – steroid hormones and other lipid molecules involved in human reproduction. Yen & Jaffe’s reproductive endocrinology : physiology, pathophysiology, and clinical management. Philadelphia, PA, Elsevier. [Google Scholar]
- Svenvik M, Raffetseder J, Brudin L, Lindberg R, Blomberg M, Axelsson D, Jenmalm MC, Ernerudh J and Nording ML (2021). “Plasma oxylipin levels associated with preterm birth in preterm labor().“ Prostaglandins Leukot Essent Fatty Acids 166: 102251. [DOI] [PubMed] [Google Scholar]
- Tenorio MB, Ferreira RC, Moura FA, Bueno NB, Goulart MOF and Oliveira ACM (2018). “Oral antioxidant therapy for prevention and treatment of preeclampsia: Meta-analysis of randomized controlled trials.” Nutr Metab Cardiovasc Dis 28(9): 865–876. [DOI] [PubMed] [Google Scholar]
- Tetteh PW, Adu-Bonsaffoh K, Antwi-Boasiako C, Antwi DA, Gyan B and Obed SA (2015). “Assessment of Oxidative Stress in Early and Late Onset Pre-Eclampsia among Ghanaian Women.” J West Afr Coll Surg 5(1): 42–58. [PMC free article] [PubMed] [Google Scholar]
- Thompson LP and Al-Hasan Y (2012). “Impact of oxidative stress in fetal programming.” J Pregnancy 2012: 582748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traber MG and Stevens JF (2011). “Vitamins C and E: beneficial effects from a mechanistic perspective.” Free Radic Biol Med 51(5): 1000–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turpin CA, Sakyi SA, Owiredu WK, Ephraim RK and Anto EO (2015). “Association between adverse pregnancy outcome and imbalance in angiogenic regulators and oxidative stress biomarkers in gestational hypertension and preeclampsia.” BMC Pregnancy Childbirth 15: 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van’t Erve TJ, Lih FB, Kadiiska MB, Deterding LJ, Eling TE and Mason RP (2015). “Reinterpreting the best biomarker of oxidative stress: The 8-iso-PGF(2alpha)/PGF(2alpha) ratio distinguishes chemical from enzymatic lipid peroxidation.” Free Radic Biol Med 83: 245–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van TETJ, Rosen EM, Barrett ES, Nguyen RHN, Sathyanarayana S, Milne GL, Calafat AM, Swan SH and Ferguson KK (2019). “Phthalates and Phthalate Alternatives Have Diverse Associations with Oxidative Stress and Inflammation in Pregnant Women.” Environ Sci Technol 53(6): 3258–3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderweele TJ (2015). Explanation in causal inference: Methods for mediation and interaction. New York, NY, US, Oxford University Press. [Google Scholar]
- Varshavsky J, Smith A, Wang A, Hom E, Izano M, Huang H, Padula A and Woodruff TJ (2020). “Heightened susceptibility: A review of how pregnancy and chemical exposures influence maternal health.” Reprod Toxicol 92: 14–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vawda S, Mansour R, Takeda A, Funnell P, Kerry S, Mudway I, Jamaludin J, Shaheen S, Griffiths C and Walton R (2014). “Associations between inflammatory and immune response genes and adverse respiratory outcomes following exposure to outdoor air pollution: a HuGE systematic review.” Am J Epidemiol 179(4): 432–442. [DOI] [PubMed] [Google Scholar]
- Villar J, Purwar M, Merialdi M, Zavaleta N, Thi Nhu Ngoc N, Anthony J, De Greeff A, Poston L, Shennan A, W. H. O. V. C. and E. t. g. Vitamin (2009). “World Health Organisation multicentre randomised trial of supplementation with vitamins C and E among pregnant women at high risk for pre-eclampsia in populations of low nutritional status from developing countries.” BJOG 116(6): 780–788. [DOI] [PubMed] [Google Scholar]
- Vinding RK, Stokholm J, Sevelsted A, Chawes BL, Bonnelykke K, Barman M, Jacobsson B and Bisgaard H (2019). “Fish Oil Supplementation in Pregnancy Increases Gestational Age, Size for Gestational Age, and Birth Weight in Infants: A Randomized Controlled Trial.” J Nutr 149(4): 628–634. [DOI] [PubMed] [Google Scholar]
- Voltolini C, Torricelli M, Conti N, Vellucci FL, Severi FM and Petraglia F (2013). “Understanding spontaneous preterm birth: from underlying mechanisms to predictive and preventive interventions.” Reprod Sci 20(11): 1274–1292. [DOI] [PubMed] [Google Scholar]
- Wang T, Han Y, Li H, Wang Y, Xue T, Chen X, Chen W, Fan Y, Qiu X, Gong J, Xu Y, Wang J, Li W and Zhu T (2021a). “Changes in bioactive lipid mediators in response to short-term exposure to ambient air particulate matter: A targeted lipidomic analysis of oxylipin signaling pathways.” Environ Int 147: 106314. [DOI] [PubMed] [Google Scholar]
- Wang W, Xie X, Yuan T, Wang Y, Zhao F, Zhou Z and Zhang H (2021. b). “Epidemiological trends of maternal hypertensive disorders of pregnancy at the global, regional, and national levels: a population-based study.” BMC Pregnancy Childbirth 21(1): 364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins DJ, Ferguson KK, Anzalota Del Toro LV, Alshawabkeh AN, Cordero JF and Meeker JD (2015). “Associations between urinary phenol and paraben concentrations and markers of oxidative stress and inflammation among pregnant women in Puerto Rico.” Int J Hyg Environ Health 218(2): 212–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch B, Keil A, van ‘t Erve T, Deterding L, Williams J, Lih F, Cantonwine D, McElrath T and Ferguson K (2020a). “Longitudinal profiles of plasma eicosanoids during pregnancy and size for gestational age at delivery: a nested case-control study.” PLoS Medicine 17(8):e1003271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch BM, Branscum A, Geldhof GJ, Ahmed SM, Hystad P, Smit E, Afroz S, Megowan M, Golam M, Sharif O, Rahman M, Quamruzzaman Q, Christiani DC and Kile ML (2020b). “Evaluating the effects between metal mixtures and serum vaccine antibody concentrations in children: a prospective birth cohort study.” Environ Health 19(1): 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch BM, Keil AP, Bommarito PA, van T’ Erve TJ, Deterding LJ, Williams JG, Lih FB, Cantonwine DE, McElrath TF and Ferguson KK (2021). “Longitudinal exposure to consumer product chemicals and changes in plasma oxylipins in pregnant women.” Environ Int 157: 106787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff TJ, Zota AR and Schwartz JM (2011). “Environmental chemicals in pregnant women in the United States: NHANES 2003-2004.” Environ Health Perspect 119(6): 878–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CC, Gupta T, Garcia V, Ding Y and Schwartzman ML (2014). “20-HETE and blood pressure regulation: clinical implications.” Cardiol Rev 22(1): 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Cai H, Xiang YB, Cai Q, Yang G, Liu D, Sanchez S, Zheng W, Milne G and Shu XO (2010). “Intra-person variation of urinary biomarkers of oxidative stress and inflammation.” Cancer Epidemiol Biomarkers Prev 19(4): 947–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagel S, Cohen SM and Goldman-Wohl D (2021). “An integrated model of preeclampsia: a multifaceted syndrome of the maternal cardiovascular-placental-fetal array.” Am J Obstet Gynecol. [DOI] [PubMed] [Google Scholar]
- Yu H, Yin Y, Zhang J and Zhou R (2020). “The impact of particulate matter 2.5 on the risk of preeclampsia: an updated systematic review and meta-analysis.” Environ Sci Pollut Res Int 27(30): 37527–37539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoeller RT, Brown TR, Doan LL, Gore AC, Skakkebaek NE, Soto AM, Woodruff TJ and Vom Saal FS (2012). “Endocrine-disrupting chemicals and public health protection: a statement of principles from The Endocrine Society.” Endocrinology 153(9): 4097–4110. [DOI] [PMC free article] [PubMed] [Google Scholar]


