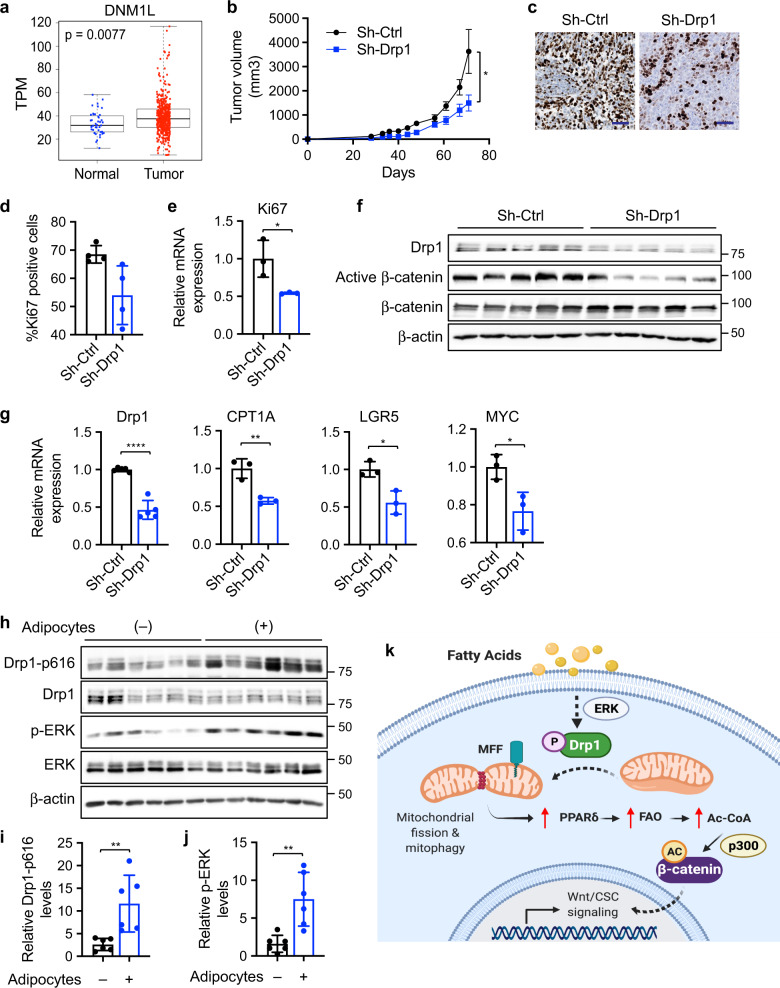
Fig. 7. Knockdown of Drp1 inhibits xenograft tumor growth and Wnt signaling in vivo.
a Bioinformatic analysis of TCGA-COAD dataset showed higher Drp1 (DNM1L gene) expression in tumors compared to normal samples (p = 0.0077 based on linear mixed models). b Sh-Ctrl and sh-Drp1 PT130 cells were injected subcutaneously into NSG mice. The size of tumors was measured every 3–5 days starting at the 3rd week after injection. Data were presented as mean ± SEM (n = 12, *p < 0.05). c Representative images from tumor sections stained with the Ki67 antibody. Scale bar, 25 μm. d The percentage of Ki67 positive cells were quantified in tumors from 4 mice of each group using HALO. Data were presented as mean ± SD (n = 4, *p < 0.05). e Tumor tissues from three mice of each group were analyzed for the expression of Ki67 mRNA using RT-qPCR. Data were presented as mean ± SD (n = 3, *p < 0.05). f Tumor tissues from sh-Ctrl and sh-Drp1 group were analyzed for the expression of Drp1, active β-catenin, total β-catenin, and β-actin using western blot. g Tumor tissues from three mice of each group were analyzed for the expression of Drp1, CPT1A, LGR5, and MYC mRNA using RT-qPCR. Data were presented as mean ± SD (n = 3, *p < 0.05, **p < 0.01, and ****p < 0.0001). h Suspensions of SW480 cells with or without adipocytes were injected subcutaneously into NSG mice and tumor tissues were analyzed for the expression of Drp1-p616, Drp1, p-ERK, ERK, and β-actin using western blot. i, j Relative Drp1-p616 (i) and p-ERK (j) levels were quantified by normalizing Drp1-p616 to Drp1 and p-ERK to ERK, respectively. Data were presented as mean ± SD (n = 6, **p < 0.01). k Results from our study support a model in which fatty acids activate Drp1/MFF-mediated mitochondrial fission to promote Wnt/β-catenin signaling by remodeling cellular metabolic pathways.