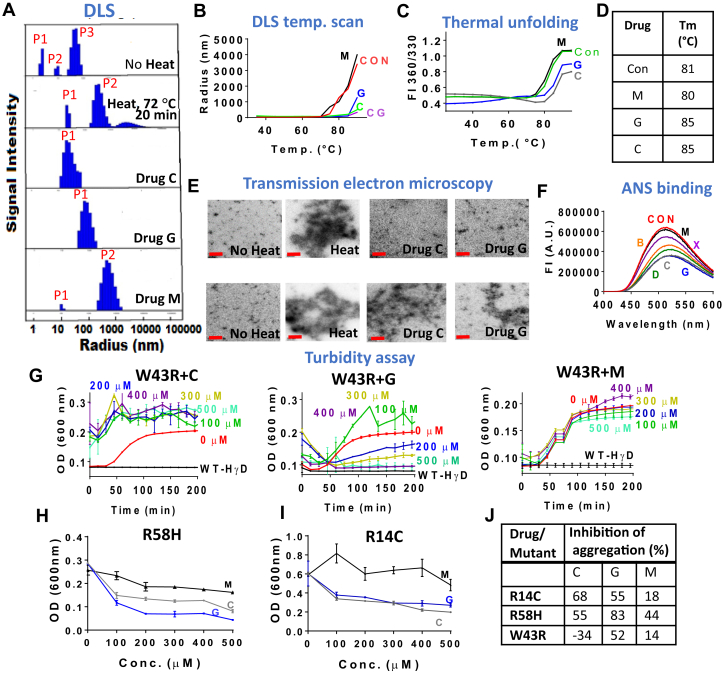
Figure 2.
Inhibitory effect of closantel (C) and gambogic acid (G) against heat-induced aggregation of HγD crystallin and its mutants. Chaulmoogric acid (drug M) served as negative control. A, dynamic light scattering spectra of thermally stressed (72 °C, 20 min) WT-HγD crystallin (50 μM in 50 mM K3PO4 buffer, pH 7.2) alone and with drugs C, G, and M (400 μM in dimethyl sulfoxide [DMSO]). B, DLS temperature scan of 50 μM of WT-HγD samples with and without drug C, G, and equimolar CG together. Samples were heated from 30 °C to 90 °C with 1 °C/min rise in temperature. C, thermal unfolding study of 10 μM WT-HγD crystallin with or without 100 μM of drug C, G, and M in DMSO. D, table showing Tm of WT-HγD alone and with drugs C, G, and M. E, transmission electron micrographs of WT-HγD (50 μM) heated with and without drugs C and G (400 μM). Two different images are presented for each condition of the same samples. The red bars indicate 200 nm scale. F, bis-ANS binding study of 10 μM WT-HγD with and without 100 μM of drugs (C, G, X, D, B, and M). G, effects of drugs C, G, and M (0–500 μM) on turbidity assay (kinetic study at 42 °C) of W43R mutant (43 μM in 50 mM K3PO4 buffer) versus WT-HγD. The readings were taken at an interval of every 10 min for a total of 200 min. End-point turbidity assay (thermal stress at 72 °C for 20 min) of R58H (H) and R14C (I) mutants of WT-HγD (50 mM K3PO4 buffer, 5 mM DTT) at different drug concentrations of C, G, and M. J, table showing percent inhibition of aggregation of mutants by 500 μM of the drugs (see Experimental procedures section for details). Each experiment was done in triplicates, and the results represent average ± std dev. of three individual readings. bis-ANS, bis-8-anilino-1-naphthalene sulfonic acid; DLS, dynamic light scattering. HγD, human gamma D.