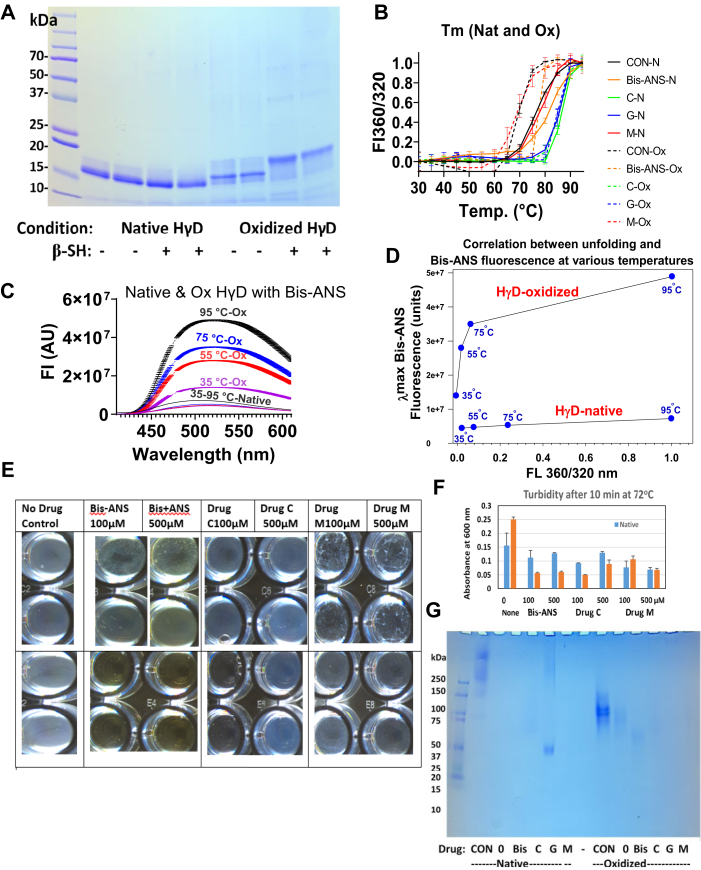
Figure 3.
Comparative studies of native and oxidized HyD.A, SDS gel of native and oxidized HγG with and without reduction with β-mercaptoethanol showing no effect of reduction on the native form of HyD but upward migration shift of oxidized HγD. B, comparative effects of drugs C, G, and M on Tm of native and oxidized HyD showing HyD-ox unfolds at lower temperature than native HyD with both drugs C and G, as well as bis-ANS, except M, improving the Tm of both proteins. C, bis-ANS fluorescence of native HγD barely increases with thermal stress, whereas marked increase is noted with the oxidized protein. D, correlation between unfolding based on 360/320 nm fluorescence ratio and bis-ANS fluorescence shows that bis-ANS fluorescence dramatically increases with moderate unfolding of HγD-ox, whereas relatively little bis-ANS binding is observed during unfolding of native HγD. E and F, effect of 100 and 500 μM bis-ANS and drugs C and M on absorbance at 600 nm (F) and dark field microscopic images (E) of native and oxidized HγD (1.4 mg/ml with DTPA) incubated for 10 min at 72 °C. The images of the microtiter wells reveal intense milky appearance of the control sample that was strongly suppressed by both 100 and 500 μM bis-ANS, 100 μM drug C, and 500 μM drug M. Note the presence of coalesced insoluble aggregates in the native protein incubated with drug M suggesting caution with the interpretation of turbidity data without morphological data. G, native PAGE of native and oxidized nonheated HγD protein (CON) and protein heated at 72 °C for 10 min without (0) or with 100 μM bis-ANS, C, G, and M drugs reveals no protein stain, likely because of blocking of Coomassie binding sites by the drugs except for drug G. bis-ANS, bis-8-anilino-1-naphthalene sulfonic acid; DTPA, diethylenetriaminepentaacetic acid; HγD, human gamma D.