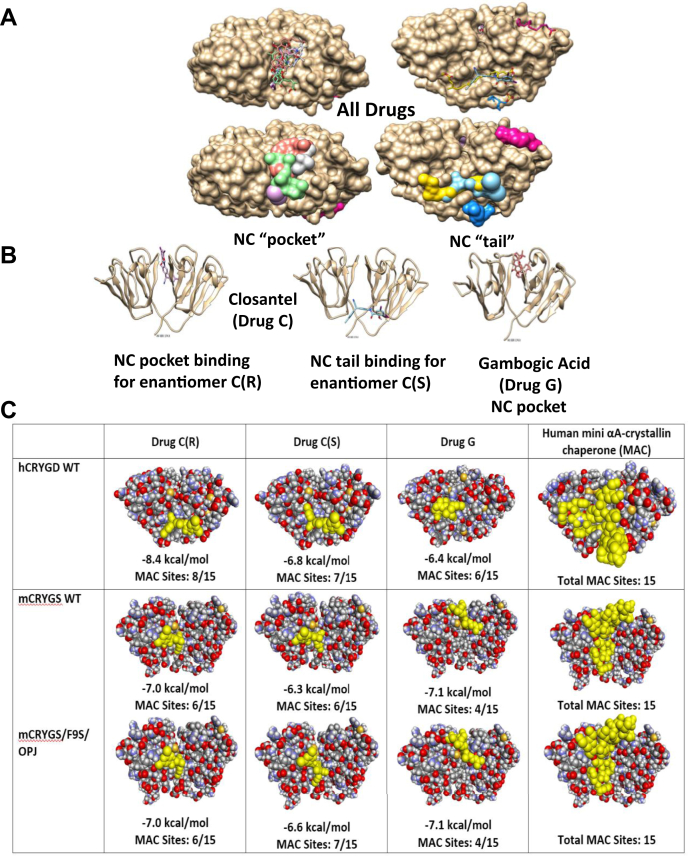
Figure 4.
Graphic rendition of the interactions. Interactions between (A) the six candidate drugs listed in Table 1 with hγD space-fill model obtained by molecular docking. B, the enantiomers of drug C(S) and C(R) and drug G with the ribbon model of HγD. C, the binding sites of drugs C and G to HγD and MγS compared with the binding sites of the mini-αA-crystallin chaperone (MAC) peptide “KFVIFLDVKHFSPEDLTVK” obtained by molecular docking using Autodock Vina software. The N-terminal domain of the gamma crystallin models is on the left. A shows that most drugs bind either in the NC pocket or NC tail of the protein, and B shows that drugs C and G can bind both sites. Drug M (magenta) and drug T (dark blue) that had no protein stabilizing activity bind only to the C domain (M) or at the tip of the molecule (T), respectively. C shows significant overlap between the binding regions for drugs C and G onto human and mouse CRYGD and CRYGS and its mutant CRYGS/F9S. The number of shared binding sites with the 15 MAC sites are provided. Computed binding affinities are listed in kilocalorie/mole. Information on specific binding sites is provided in Tables 5 and 6. HγD, human gamma D.