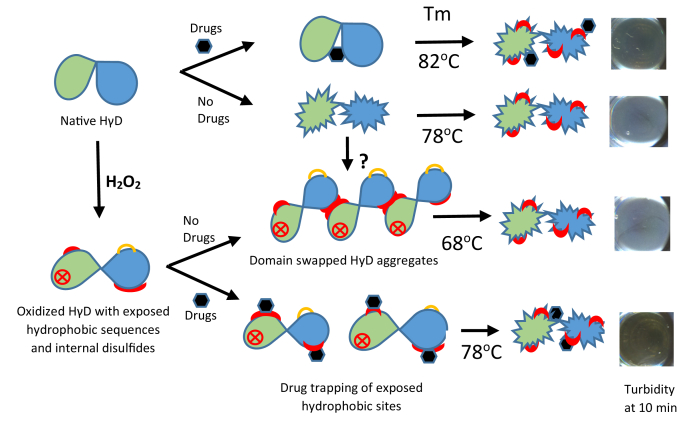
Figure 5.
Tentative mechanism by which the α-crystallin mimetic drugs might prevent solution turbidity of oxidized and native HγD using bis-ANS as a proxy for the drugs based on our findings that it potently suppresses heat-induced turbidity. A mechanism based on domain swapping (74) and exposure of hydrophobic bis-ANS binding sequences is proposed. In the oxidized protein, the latter were mostly exposed already below the Tm of HγD-ox, whereas no such exposure was noted for HγD-nat until the Tm was reached. Yet in both cases, bis-ANS, like drugs G and C, was able to increase the Tm of both oxidized and native HγD. Based on Serebryany’s work, internal disulfides are likely present in HγD-ox (74). However, aggregates seen by native PAGE (Fig. 3G) are unlikely crosslinked by external disulfides since these were absent from HγD-ox by SDS-PAGE. Extensive studies will be needed to probe the aforementioned tentative mechanisms and role of aggregates in light of current knowledge of factors that impact on the stability of HγD N- and C-terminal domains. Legend: red circle with cross: generic oxidative damage; red color: postulated exposed hydrophobic domains; orange half-circles: internal disulfides; and black six-membered ring: drug. Note that the images showing the presence or absence of protein aggregates at the bottom of the wells are identical to those of Figure 3E. They are being reused here for the sake of linking the proposed aggregation mechanism with a real instead of a simulated picture. bis-ANS, bis-8-anilino-1-naphthalene sulfonic acid; HγD, human gamma D.