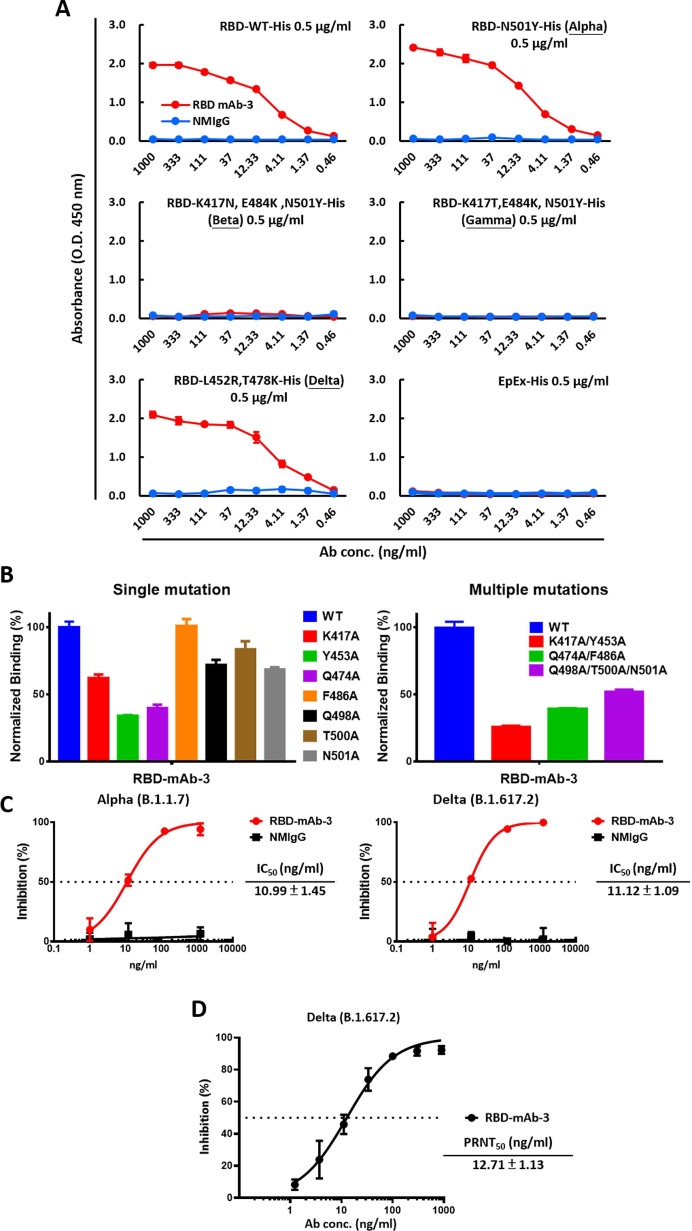
Fig. 5.
RBD-mAb-3 exhibits potent binding and neutralization of SARS-CoV-2 Delta. (A) The binding of RBD-mAb-3 to different variants of SARS-CoV-2 RBDs was determined by ELISA analysis. EpEX served as a negative control. (B) Epitope mapping of RBD-mAb-3 using mutagenesis. RBD-mAb-3 binding to mutant RBDs with single or multiple alanine mutations was normalized to its binding to wild-type (WT) RBD; measurements were made with cellular ELISA. (C) Neutralization assays were performed on SARS-CoV-2 Alpha and Delta pseudoviruses with purified RBD-mAb-3. Assays were performed in triplicate; each point represents the mean ± SEM. IC50 values were calculated with GraphPad Prism software. (D) RBD-mAb-3 neutralizes SARS-CoV-2 Delta according to PRNT. The PRNT50 value was calculated with GraphPad Prism software. Assays were performed in triplicate, and points represent the mean ± SD.