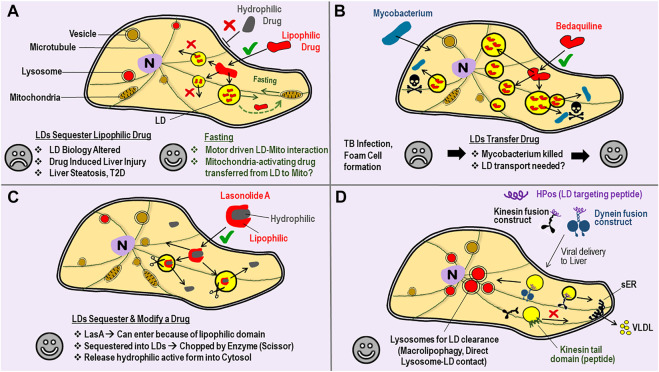
FIGURE 2.
Lipid droplets can Sequester, Transfer or Modify Drugs–perhaps one could achieve targeted Drug Delivery by manipulating Motors on LDs. (A) A cartoon view of a Cell. Bilayer lipid membranes are shown with double-lines (e.g., plasma membrane, vesicle membrane) to distinguish from the monolayer lipid membrane on Lipid droplets (LDs). Entry into cells through the plasma membrane is often easier for lipophilic drugs (√ sign) as compared to hydrophilic drugs (× sign). Once inside, the lipophilic drug is sequestered away into LDs from where it cannot escape into cytosol, and therefore becomes ineffective as a drug. Such drug sequestration can lead to some of the undesirable consequences listed (sad face). Fasting/Starvation is known to increase LD-mitochondria interaction and consumption of LD-contents by mitochondrial β-oxidation. Perhaps hydrophobic drugs that can reverse mitochondrial degeneration can be packaged into LDs, and then delivered to mitochondria with a desirable outcome (happy face). (B) Mycobacterium tuberculosis infection causes LD accumulation (foam cell formation) in macrophages, with lipid-rich granulomas serving as a nutrient source for the bacteria. Bedaqulinine, a hydrophobic anti-tubercular drug, is sequestered away into LDs. The LDs act as a transferable reservoir of bedaquiline to kill Mycobacterium (skull sign). This function of LDs is in contrast to the sequestration of drugs by LDs described in panel-A. Perhaps LD-bacteria interactions (and therefore bedaquiline delivery) can be enhanced by targeted manipulation of motor proteins on the monolayer LD membrane. (C) Lasonolide-A (LasA) has both lipophilic and hydrophobic domains. LasA therefore enters through the cell membrane and accumulates into LDs, but there an enzyme (scissor) removes the hydrophobic part. Cleaved LasA is released into cytosol as a potent anti-cancer agent. This opens the possibility of designer drugs paired with cognate enzymes on LDs that can possibly be much more effective. (D) Targeted removal of kinesin-1 from LDs by using a kinesin tail domain peptide has been demonstrated (see main text). This intervention specifically blocks the transport of LDs to peripheral regions of hepatocytes inside the liver, so that the LDs can no more interact with the smooth-ER (sER). This treatment reduces serum triglycerides in the animal by reducing the triglyceride content of VLDL particles secreted from the liver. LD-targeting peptides (e.g., HPos) are known. HPos fusion constructs with motors can be targeted to the liver by adenoviral delivery, so that the motors accumulate specifically to LDs inside liver cells. A kinesin fusion construct could deliver LDs to the peripheral sER for increased clearance of LDs (via VLDL secretion) to ameliorate fatty liver conditions. A dynein construct could deliver LDs to lysosomes for LD-clearance via autophagic pathways.