Figure 5. FIGURE 5: Mechanistic model for holin-mediated non-lytic transport of proteins.
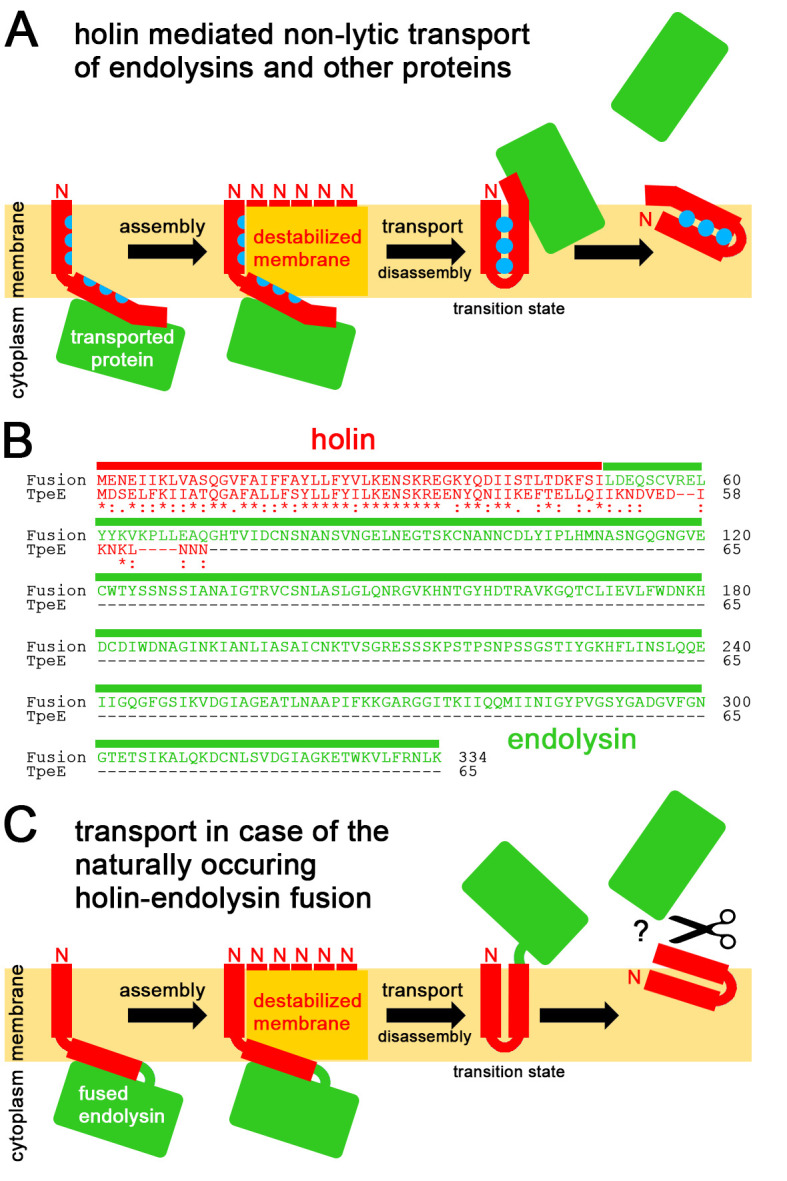
(A) We propose the following mechanism: Transported proteins associate with the cytoplasmically exposed APH/C-terminus region, holins assemble to multimeric patches that locally destabilize the membrane by membrane-thinning as in case of TatA (assembly; note that for clarity reasons only the multiple N-termini of the associated protomers are indicated), the hydrophobic face of individual substrate-associated APHs in these multimeric holin patches flip at these destabilized membrane sites irreversibly into a trans-membrane orientation, triggered by interactions with the TMH, thereby moving the associated protein through the destabilized membrane (transport), the interacting TMH and APH are now not anymore stably trans-membrane oriented and dissociate from the other holin protomers (disassembly), resulting in an unstable transition state as the hydrophilic face of the APH will readily move to the membrane surface, finally resulting in release of the transported protein from the trans-side of the membrane. (B) Sequence and domain organization of the natural holin-endolysin fusion, and alignment with C. perfringens TpeE. Holin sequences are in red, the fused endolysin sequence is in green. (C) Translocation mechanism as expected for the fusion protein. The mechanism as described in (A) is strongly supported by the natural holin-endolysin fusion. The fused endolysin might need to be cleaved off to function. See text for details.
