Abstract
Purpose
Pyrotinib, a novel human epidermal growth factor receptor 2 (HER2)-targeted tyrosine kinase inhibitor (TKI), has led to remarkable survival outcomes in HER2-positive advanced breast cancer (ABC) in clinical trials and was approved for second-line standards of treatment for HER2+ ABC in China. However, the clinical trials could not fully reflect reality of clinical practice, and predictive factors were still lacking. This study aimed to assess the actual efficacy and safety of pyrotinib in HER2+ ABC in real-world setting.
Patients and Methods
In this multicenter, retrospective, observational real-world study, we analyzed 171 patients with HER2+ ABC, who received pyrotinib-based treatment from November 2017 to November 2020. The primary end point was progression-free survival (PFS). Secondary end points included overall survival (OS), objective response rate (ORR), clinical benefit rate (CBR) and safety.
Results
Up to November 30, 2021, the median PFS (mPFS) was 12.0 months for all patients. One hundred and sixty-two patients (94.7%) with measurable lesions had been included in efficacy assessment. The ORR and CBR were 45.1% and 81.5%, respectively. A significantly longer PFS was reported in patients who received pyrotinib as first-line treatment, had the ECOG-PS of 0–1, as well as those who were lapatinib-naive. In addition, multivariable analysis indicated that ECOG-PS of 2–4, positive hormone receptor (HR) status, and presence of visceral metastasis were independent negative predictors of PFS. As far as we know, this study first reported the survival outcome of pyrotinib cross-line treatment, with a mPFS of 5.0 months. All grades of adverse events (AEs) occurred in 171 patients (100%), and the most common AE was diarrhea (86.5%).
Conclusion
This study further demonstrated the outstanding efficacy and safety of pyrotinib and reported the potential predictors of survival in HER2+ ABC.
Keywords: breast cancer, tyrosine kinase inhibitors, prognostic factor, targeted therapy
Introduction
Breast cancer is the most commonly diagnosed female cancer worldwide, of which approximately 20% were characterized by HER2 overexpression, leading to aggressive clinical behaviors and poor outcomes.1,2 Multiple HER2-targeted agents, such as trastuzumab, pertuzumab, lapatinib, as well as T-DM1, were developed to improve the outcomes of HER2+ ABC.2,3 Despite significant breakthroughs in HER2-targeted therapies, the popularization of some anti-HER2 agents is limited in part of the world owing to their scarce accessibility. In addition, many patients develop primary/de-novo or secondary resistance against anti-HER2 agents, which is a barrier to health promotion.4 Thus, it is imperative to explore novel HER2-targeted strategies to overcome the clinical dilemma in treatment of HER2+ ABC.
Pyrotinib is a small-molecule irreversible TKI targeting HER1, HER2, and HER4.5 A massive number of studies have reported the therapeutic efficacy of pyrotinib in HER2+ ABC. In phase 1 study, patients with heavily-treated HER2+ ABC could benefit from pyrotinib-based treatment whether administered alone or combined with capecitabine.5 In phase 2 study, pyrotinib plus capecitabine significantly improved the PFS (18.1 months vs 7.0 months, p<0.001) and ORR (78.5% vs 57.1%, p=0.01) compared with those receiving lapatinib and capecitabine in second-line treatment of HER2+ ABC.6 Furthermore, in phase 3 PHENIX study, pyrotinib plus capecitabine significantly prolonged PFS (11.1 months vs 4.1 months, p<0.001) compared with capecitabine monotherapy in patients with HER2+ local-relapsed or metastatic breast cancer, who had received trastuzumab and taxanes.7 Moreover, the PHOEBE trial revealed that pyrotinib plus capecitabine significantly improved PFS (12.5 months vs 6.8 months, p<0.0001) when compared with lapatinib plus capecitabine in patients with metastatic breast cancer.8 Recently, the phase 2 PERMEATE study further demonstrated that pyrotinib-based treatment was a potential therapeutic option for HER2+ ABC patients with brain metastases (BM).9 Based on these remarkable results, in July 2020, pyrotinib was approved for second-line standards of treatment for HER2+ ABC by National Medical Products Administration of China. However, the clinical trials could not fully reflect reality of clinical practice, and predictive factors were still lacking.
This multicenter, retrospective, real-world study aimed to evaluate the efficacy and safety of pyrotinib in HER2+ ABC in real clinical practice, so as to provide more therapeutic information about pyrotinib to achieve accurate treatment.
Patients and Methods
Study Design
This is a multicenter, retrospective, observational, real-world study. Between November 2017 and November 2020, a total of 171 patients with HER2+ ABC were included from 7 hospitals in China. The data-cutoff date for analysis was November 30, 2021. This trial had been registered in Chinese Clinical Trial Registry (ChiCTR2100051632).
Patients and Treatments
Eligible patients were women ≥18 years of age. Patients should have pathologically confirmed ABC that had been determined to be HER2+ on the basis of a score of 3+ by immunohistochemistry (IHC), or gene amplification by fluorescence in-situ hybridization (FISH) with IHC score of 2+.10 All assessments were confirmed at their own hospitals. Patients should have complete medical records reviewed for clinical data. Patients were excluded if they had been exposed to pyrotinib, had any serious systemic disorders, or were pregnant or lactating.
All patients were administrated pyrotinib orally in a 21-day cycle. The starting dose, dose modification, dose interruption, treatment discontinuation, and combinational strategies were determined at the physicians’ discretion according to clinical manifestations.
Primary resistance to trastuzumab was defined as progression at first radiological reassessment at 8–12 weeks or within 3 months after first-line trastuzumab in the metastatic setting or new recurrences diagnosed during or within 12 months after adjuvant trastuzumab.
Outcomes
The primary end point was PFS (defined as the duration from treatment initiation to disease progression or death due to any cause). The secondary end points included OS (defined as the time from treatment initiation to death resulting from any reason), ORR (defined as the percentage of patients with measurable disease who had a complete response or partial response), CBR (defined as the percentage of patients with measurable disease who had a complete response, partial response, or stable disease lasting ≥24 weeks), and safety. The efficacy was evaluated using Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. Besides, the safety was assessed according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events (NCI CTCAE) version 4.03.
Statistical Analysis
Patient characteristics were summarized by median with range for continuous variables and frequency with percentage for categorical variables. PFS and OS were calculated using the Kaplan-Meier method and compared using the Log rank test. Cox proportional hazards regression models were used to estimate hazard ratios for progression/death with 95% confidence intervals (CIs). Statistical Package for Social Sciences (SPSS) version 26.0 was used to perform statistical analysis. Data visualization was performed using R version 4.1.1. All statistical tests were two-tailed, and p<0.05 was considered statistically significant.
Results
Baseline Characteristics and Treatments
A total of 171 eligible patients were included in this study (Figure 1). The baseline characteristics were shown in Table 1. Median age was 56 years (range 29–92). Visceral metastasis accounted for 73.1%. Moreover, 166 patients (97.1%) had been previously treated with trastuzumab, of whom 82 (48.0%) were primarily resistant to trastuzumab. In addition, 41 patients (24.0%) had been exposed to lapatinib, 19 (11.1%) exposed to pertuzumab, and 6 (3.5%) exposed to T-DM1.
Figure 1.
Flow chart of the study.
Table 1.
Baseline Characteristics
| Characteristic | Total (N=171) |
|---|---|
| Median age (range), years | 56 (29–92) |
| ECOG performance-status | |
| 0–1 | 139 (81.3%) |
| 2–4 | 32 (18.7%) |
| Hormone receptor status | |
| ER or PR positive | 91 (53.2%) |
| ER and PR negative | 80 (46.8%) |
| HER2 status | |
| IHC 3+ | 139 (81.3%) |
| IHC 2+, FISH+ | 32 (18.7%) |
| Visceral metastases | |
| Yes | 125 (73.1%) |
| No | 46 (26.9%) |
| Metastatic sites | |
| Liver | 63 (36.8%) |
| Lung | 63 (36.8%) |
| Brain | 52 (30.4%) |
| Bone | 66 (38.6%) |
| Previous HER2-targeted treatments | |
| Trastuzumab | 166 (97.1%) |
| In (neo)adjuvant setting | 73 (42.7%) |
| For advanced disease | 116 (67.8%) |
| Both | 23 (13.5%) |
| Primary resistance to trastuzumab† | 82 (48.0%) |
| Lapatinib | 41 (24.0%) |
| Pertuzumab | 19 (11.1%) |
| T-DM1 | 6 (3.5%) |
Notes: †Primary resistance to trastuzumab was defined as progression at first radiological reassessment at 8–12 weeks or within 3 months after first-line trastuzumab in the metastatic setting or new recurrences diagnosed during or within 12 months after adjuvant trastuzumab.
Abbreviations: ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2; IHC, immunohistochemistry; FISH, fluorescence in-situ hybridization.
Treatment administration was shown in Table 2. Forty-six (26.9%) patients received pyrotinib as first-line treatment, while 125 (73.1%) as second-line or beyond treatment. The starting dose of pyrotinib was 400mg/day in 100 patients (58.5%), 320mg/day in 66 patients (38.6%), and 240mg/day in 4 patients (2.3%). A 92-year-old patient was treated with pyrotinib at 160mg/day. Moreover, one hundred and fifty patients (87.7%) received the treatment of pyrotinib in combination with chemotherapy. The most common chemotherapeutic agent was capecitabine (64.9%). Besides, two (1.2%) patients received pyrotinib plus trastuzumab with or without chemotherapy. Nine (5.3%) patients received pyrotinib monotherapy. None of them received the immunotherapy.
Table 2.
Treatment Administration
| Pyrotinib Treatment | Total (N=171) |
|---|---|
| Lines of pyrotinib-based therapy | |
| 1 | 46 (26.9%) |
| ≥2 | 125 (73.1%) |
| Starting dose of pyrotinib (mg/day) | |
| 400 | 100 (58.5%) |
| 320 | 66 (38.6%) |
| 240 | 4 (2.3%) |
| 160 | 1 (0.6%) |
| Treatment regimens | |
| Pyrotinib + chemotherapy | 150 (87.7%) |
| Pyrotinib + capecitabine | 111 (64.9%) |
| Pyrotinib + others | 39 (22.8%) |
| Pyrotinib + endocrine therapy | 10 (5.8%) |
| Pyrotinib + trastuzumab | 1 (0.6%) |
| Pyrotinib + trastuzumab + chemotherapy | 1 (0.6%) |
| Pyrotinib monotherapy | 9 (5.3%) |
Efficacy in All Patients
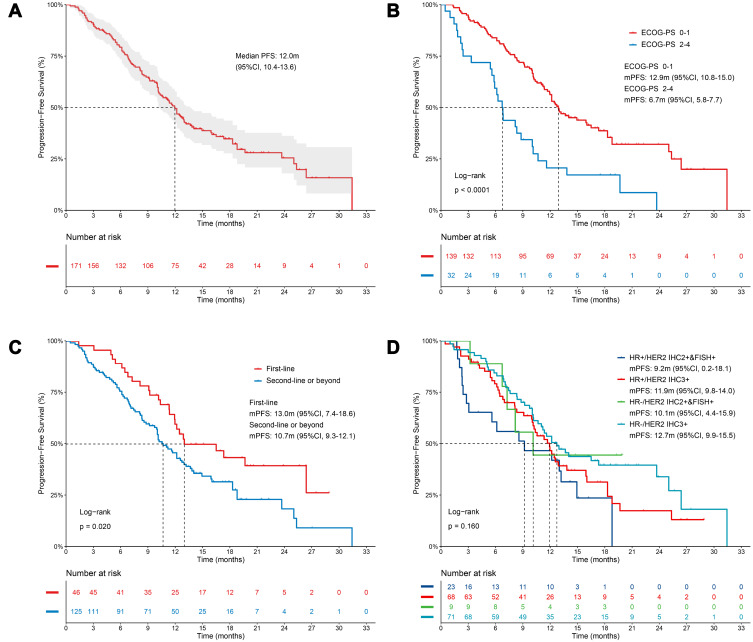
Up to November 30, 2021, the median follow-up was 17.9 months (95% CI, 16.4–19.3). The mPFS was 12.0 months (95% CI, 10.4–13.6) with 112 (65.5%) events of PFS (Figure 2A). Meanwhile, the OS data was still immature.
Figure 2.
Kaplan-Meier estimates of progression-free survival. (A) Survival analysis for all patients (n=171). (B) Survival analysis according to ECOG PS. (C) Survival analysis according to the lines of pyrotinib-based therapy. (D) Survival analysis according to the status of HR and HER2. P-values are from univariate Log rank tests.
A total of 162 (94.7%) patients with measurable lesions had been included in efficacy assessment. The ORR and CBR were 45.1% and 81.5%, respectively. Four (2.5%) patients achieved complete response, 69 (42.6%) patients achieved partial response, 73 (45.1%) patients had stable disease, and 16 (9.9%) patients achieved progressive disease. In addition, 59 (36.4%) patients had stable disease ≥ 24 weeks (Table 3).
Table 3.
Tumor Response in 162 Patients with Measurable Disease
| Best Overall Response | Total (N=162) |
|---|---|
| Complete response | 4 (2.5%) |
| Partial response | 69 (42.6%) |
| Stable disease | 73 (45.1%) |
| ≥ 24 weeks | 59 (36.4%) |
| Progressive disease | 16 (9.9%) |
| Objective response rate | 45.1% |
| Clinical benefit rate | 81.5% |
Subgroup analysis indicated that, the mPFS in patients with ECOG-PS of 0–1 was longer (12.9 months vs 6.7 months, p<0.0001) than those with ECOG-PS of 2–4 (Figure 2B). In addition, the mPFS in first-line and second-line or beyond treatment were 13.0 months and 10.7 months (p=0.020), respectively (Figure 2C).
In patients with HR- (12.2 months vs 11.7 months, p=0.056) (Supplementary Figure 1A), or HER2 IHC3+ (12.2 months vs 9.2 months, p=0.239) (Supplementary Figure 1B), there were only numerical prolonged PFS without statistical significance. Furtherly, patients were grouped into four subtypes according to the status of HR and HER2. The mPFS in patients with HR-/HER2 (IHC2+and FISH+) ABC, HR-/HER2 (IHC3+) ABC, HR+/HER2 (IHC2+and FISH+) ABC, and HR+/HER2 (IHC3+) ABC were 10.1 months, 12.7 months, 9.2 months, and 11.9 months, respectively. Of note, patients with HR-/HER2 (IHC3+) ABC had a significantly prolonged PFS (12.7 months vs 9.2 months, p=0.037) than HR+/HER2 (IHC2+and FISH+) ABC (Figure 2D).
Moreover, PFS was also improved in patients without visceral metastases (12.9 months vs 11.0 months, p=0.090) (Supplementary Figure 1C) or without bone metastases (12.2 months vs 10.1 months, p=0.070) (Supplementary Figure 1D). As for treatment administration, patients who received pyrotinib 400mg/day showed a significantly longer PFS (13.0 months vs 10.0 months, p=0.009) than those who received < 400mg/day. Furthermore, the treatment of pyrotinib plus capecitabine resulted in a longer mPFS (12.5 months vs 9.7 months, p=0.004) compared with other treatments.
Multivariable Cox regression analysis indicated that ECOG-PS of 2–4 (hazard ratio=2.84, 95% CI 1.82–4.42, p<0.001), positive hormone receptor status (hazard ratio=1.56, 95% CI 1.07–2.27, p=0.022), and presence of visceral metastasis (hazard ratio=1.74, 95% CI 1.11–2.73, p=0.016) were independent risk factors of PFS (Table 4).
Table 4.
Univariable and Multivariable Analysis of Factors Associated with PFS
| Characteristic | Univariable Analysis | Multivariable Analysis | ||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | P value | Hazard Ratio (95% CI) | P value | |
| ECOG-PS (0–1 vs 2–4) | 2.46 (1.59–3.79) | <0.001 | 2.84 (1.82–4.42) | <0.001 |
| Lines of therapy (<2 vs ≥2) | 1.68 (1.08–2.62) | 0.022 | ||
| HR status (HR- vs HR+) | 1.44 (0.99–2.10) | 0.058 | 1.56 (1.07–2.27) | 0.022 |
| HER2 status (IHC3+ vs IHC2+andFISH+) | 1.32 (0.83–2.12) | 0.242 | ||
| Visceral metastases (no vs yes) | 1.46 (0.94–2.27) | 0.093 | 1.74 (1.11–2.73) | 0.016 |
| Bone metastases (no vs yes) | 1.42 (0.97–2.07) | 0.072 | ||
Efficacy of Pyrotinib in Patients with BM
Fifty-two patients had BM at baseline, among whom 43 (82.7%) patients were trastuzumab-treated and received pyrotinib as second-line or beyond treatment. Thirty-eight patients have received brain radiotherapy. By the time of the study, 38 patients had events of PFS, resulting in a median PFS of 10.7 months (95% CI, 6.3–15.0) (Supplementary Figure 2). The 6-month and 12-month PFS rate were 74.8% and 44.3%, respectively. Additionally, there was no significantly longer PFS in patients without BM compared with those with BM (12.2 months vs 10.7 months, p=0.133).
Efficacy of Pyrotinib After Multiple HER2-Targeted Therapies
Most patients (97.1%) had received HER2-targeted therapies in this study. The treatment of pyrotinib achieved a mPFS of 11.7 months (95% CI, 10.0–13.4) in trastuzumab-treated patients.
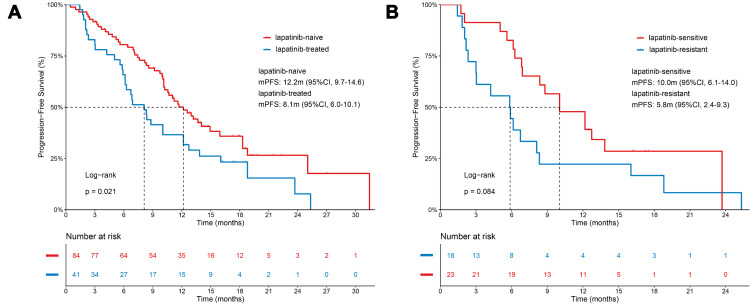
Forty-one patients (24.0%) had been treated with lapatinib for advanced disease. Survival analysis showed that, in second-line treatment or beyond, lapatinib-treated patients exhibited a significantly shorter mPFS compared to lapatinib-naive patients (8.1 months vs 12.2 months, p=0.021) (Figure 3A). Furtherly, among patients who had used lapatinib, lapatinib-sensitive patients (defined as the PFS of lapatinib ≥ 9 months) (n=23) showed a numerical longer mPFS compared with lapatinib-resistant patients (defined as the PFS of lapatinib< 9 months) (n=18) (10.0 months vs 5.8 months, p=0.084) (Figure 3B).
Figure 3.
Kaplan-Meier estimates of progression-free survival. (A) Survival analysis for patients with or without prior lapatinib exposure. (B) Survival analysis for patients who were sensitive or resistant to lapatinib. P-values are from univariate Log rank tests.
In addition, patients, who had been exposed to trastuzumab combined with pertuzumab, or T-DM1 for advanced disease, still responded to pyrotinib, with a mPFS of 9.2 months or 8.4 months, respectively.
Efficacy of Pyrotinib Cross-Line Treatments
One hundred and twelve (65.5%) patients had events of PFS, of whom 41 patients continued receiving pyrotinib while changing the combinational agents (Supplementary Table 1). The majority of them (70.7%) received the treatment of pyrotinib plus chemotherapy, and the most common chemotherapeutic agent was vinorelbine (24.4%). Besides, eight patients (19.5%) were treated with pyrotinib plus chemotherapy and targeted therapy. Survival analysis showed that patients could still benefit from the cross-line therapies of pyrotinib, achieving a mPFS of 5.0 months (95% CI, 3.8–6.3).
Safety
All grades of AEs occurred in 171 patients (100%). Grade 3–4 AEs occurred in 67 patients (39.2%). The most frequent AE was diarrhea (86.5%), and 21 (12.3%) patients experienced grade 3–4 diarrhea. Apart from diarrhea, common AEs included asthenia (71.9%), appetite loss (63.2%), dyspepsia (58.5%), and nausea (57.3%) (Table 5).
Table 5.
Pyrotinib-Related Adverse Events of All Grades and Grade 3–4
| Adverse Event | All Grades | Grade 3–4 |
|---|---|---|
| Diarrhea | 148 (86.5%) | 21 (12.3%) |
| Asthenia | 123 (71.9%) | |
| Appetite loss | 108 (63.2%) | 4 (2.3%) |
| Dyspepsia | 100 (58.5%) | |
| Nausea | 98 (57.3%) | 3 (1.8%) |
| Lymphocyte count decreased | 86 (50.3%) | 15 (8.8%) |
| Hand-foot syndrome | 83 (48.5%) | 5 (2.9%) |
| Weight loss | 73 (42.7%) | 4 (2.3%) |
| Anemia | 61 (35.7%) | 6 (3.5%) |
| White blood cell count decreased | 54 (31.6%) | 4 (2.3%) |
| Blood bilirubin increased | 53 (31.0%) | 1 (0.6%) |
| Vomiting | 51 (29.8%) | 2 (1.2%) |
Sixteen patients (9.4%) underwent dose reduction due to diarrhea (7.6%), nausea (0.6%), weight loss (0.6%), and increased blood bilirubin (0.6%), respectively. No treatment-related death or serious adverse events were reported.
In terms of hematologic toxicity, the most frequent AE was decreased lymphocyte count (50.3%). The mPFS in patients with or without decreased lymphocyte count were 12.7 months and 11.0 months (p=0.079), respectively (Supplementary Figure 3).
Discussion
Pyrotinib, a newly-developed small-molecule irreversible TKI, has attracted much attention due to its unique properties in recent years.5 This study reported the potential effects of pyrotinib in HER2-positive ABC with a mPFS of 12.0 months, similar to the outcomes in the PHENIX study (11.1 months) and PHOEBE study (12.5 months).7,8
In view of pharmacology, trastuzumab binds to the extracellular domain IV and inhibits dimerization of HER2, thus blocking signaling pathways and inhibiting cell proliferation in tumor tissues.2 Despite the significant survival benefit from trastuzumab, drug resistance was a major worldwide health challenge for HER2-positive breast cancer. Up to now, trastuzumab resistance may be associated with overexpression of p95HER2, loss of PTEN, HER2 mutations, PI3KCA mutations, overexpression of MUC4, upregulation of IGF-1R, and so on.11 Pyrotinib, a small molecule pan-HER TKI, irreversibly binds to the intracellular tyrosine kinase to inhibit tumor cell growth.5 Owing to the different anticancer mechanisms, pyrotinib allowed a valid treatment for HER2+ ABC, which was expected to overcome the trastuzumab resistance.
To date, the strategy of trastuzumab in combination with pertuzumab and docetaxel is recommended as first-line treatment in HER2+ ABC, which was approved on the basis of CLEOPATRA study.12 The study revealed that the PFS and OS were significantly improved with the treatment of trastuzumab plus pertuzumab and docetaxel versus trastuzumab plus docetaxel.12 Moreover, the PUFFIN study, a bridging study of CLEOPATRA study in China, further demonstrated the superior therapeutic outcomes of dual HER2-targeted therapy in Chinese patients.13 Our study showed that pyrotinib treatment achieved a mPFS of 13.0 months, which was comparative to the result of trastuzumab plus pertuzumab in PUFFIN study (14.5 months). On this basis, pyrotinib may have the potential to rank in first-line setting.
T-DM1, an antibody-drug conjugate, was recommended as second-line therapy after trastuzumab for HER2+ ABC.14 For trastuzumab-treated patients, the phase 3 EMILIA study indicated a significantly longer mPFS (9.6 months vs 6.4 months, p<0.001) in patients receiving T-DM1, compared to that of patients who received lapatinib plus capecitabine.15 In our study, the mPFS of pyrotinib in trastuzumab-treated patients was 11.7 months, indicating its superior to T-DM1 in EMILIA study. Despite the absence of head-to-head clinical trials, considering the therapeutic efficiency, drug accessibility, and cost in China, pyrotinib is a better choice for second-line treatment than T-DM1. Nowadays, the breast cancer guideline of Chinese Society of Clinical Oncology (CSCO) recommended pyrotinib as the preferred second-line therapy for HER2+ ABC.
For HER2+ ABC patients confirmed disease progression after multiple anti-HER2 therapies, no standard treatment is recommended at present. Neratinib, an irreversible TKI of HER1, HER2, and HER4, was approved by United States Food and Drug Administration (FDA) as an extended HER2-targeted adjuvant therapy after trastuzumab for HER2+ early breast cancer (EBC).3 Besides, neratinib was also approved in third-line HER2+ ABC.16 As we know, both neratinib and pyrotinib are irreversible TKIs of HER1, HER2, and HER4, which determines the similar properties of two drugs. In detail, both drugs are mainly metabolized by CYP3A4 in liver, and the most common AE was diarrhea (>90%).3 As for clinical trials, phase 3 NALA study compared the efficacy and safety of neratinib against lapatinib, a reversible TKI of HER1 and HER2, in patients who had received more than two lines of HER2-targeted therapies, and resulted in a significantly prolonged mPFS in the treatment of neratinib plus capecitabine (8.8 months vs 6.6 months, p=0.0059) than lapatinib plus capecitabine.16 In terms of pyrotinib, phase 3 PHOEBE study showed that the mPFS was significantly improved in the treatment of pyrotinib plus capecitabine (12.5 months vs 6.8 months, p<0.0001) than lapatinib plus capecitabine in trastuzumab-treated patients.8 However, patients enrolled in the two studies had different baseline characteristics, especially the unequal treatment lines, so the cross study comparison (12.5 months vs 8.8 months) maybe not rigorous enough. Recently, Zhang et al reported a mPFS (8.4 months) of pyrotinib in third-or-higher line treatment HER2+ ABC, which was comparable to that of neratinib (8.8 months) in NALA study.17 Unfortunately, head-to-head clinical trials were still lacking, and the direct comparison between neratinib and pyrotinib was urgently needed.
Moreover, in TH3RESA study, for patients who had received ≥2 HER2-targeted agents including trastuzumab and lapatinib, T-DM1 demonstrated a significantly improved PFS (6.2 months vs 3.3 months, p<0.0001) compared with other physician’s determinations.18 Furthermore, our study reported the mPFS of pyrotinib was 8.1 months in lapatinib-treated patients, which was better than 6.2 months in TH3RESA study. Besides, pyrotinib cross-line therapy also achieved a mPFS of 5.0 months. Considering these potential therapeutic effects, pyrotinib could emerge as a potential therapeutic strategy in refractory HER2+ ABC.
HER2+ breast cancer patients are at high risk of developing BM, with the incidence ranging from 30% to 50%, and leading to poor clinical outcomes.19 Owing to the difficulty for large molecule drugs crossing the blood-brain barrier, monoclonal antibodies are limited in HER2+ ABC patients with BM, whereas small-molecule TKIs are more permeable to control BM potentially.20 Multiple potential anticancer agents have been developed for patients with BM, including lapatinib (median time to progress, 5.5 months; median OS (mOS), 17.0 months), neratinib (mPFS, 5.5 months; mOS, 15.1 months), tucatinib (mPFS, 7.6 months; mOS, 18.1 months), and pyrotinib (mPFS, 8.7 months; mOS, 13.9 months).21–25 In our study, the mPFS of pyrotinib for patients with BM was 10.7 months, with a 6-month PFS rate of 74.8%. In conclusion, the competitive data of pyrotinib furtherly announced the efficacy of pyrotinib in BM subset.
In HER2+ breast cancer patients, more than half of the patients expressed estrogen receptor (ER) and/or progesterone receptor (PR).26,27 Multivariable analysis indicated that HR-negative patients had better PFS than HR-positive patients (12.2 months vs 11.7 months, hazard ratio=1.56, p=0.022), which may be related to the crosstalk between HER2 and HR regulating negatively.28–30 Besides, patients with visceral metastases were independently correlated with an over 1.7 times greater risk of having shorter PFS (hazard ratio=1.74; 95% CI, 1.11–2.73; p=0.016) than those without visceral metastases. Furthermore, ECOG-PS of 2–4 was also an independent risk factor for PFS (hazard ratio=2.84, 95% CI, 1.82–4.42; p<0.001), which may be explained by the special tumor burden of these patients. In detail, among patients with ECOG-PS of 2–4, seven patients (21.9%) had one metastatic organ, ten (31.3%) had two, and fifteen (46.9%) had three or more metastatic organs.
In addition, neoadjuvant treatment for EBC is also crucial.31 Recently, several studies reported the promising efficacy and manageable toxicity of pyrotinib-containing neoadjuvant therapy in HER2+ EBC, which suggested that pyrotinib could emerge as a novel option for the neoadjuvant treatment of HER2+ EBC.32,33
In terms of safety, the most common AE was diarrhea, with the incidence of 86.5%, lower than that reported in randomized controlled trials.7,8 The reason for this may be that patients in our study received the treatment of pyrotinib in a relatively low dose, with only 58.5% of patients treated with an initial dose of 400 mg/day. Furthermore, the most frequent hematologic AE was decreased lymphocyte counts (50.3%). As far as we know, it is the first time to find the potential association of clinical benefit of pyrotinib with the AE of decreased lymphocyte counts.
There were some limitations of our study. Firstly, as it is a retrospective real-world study, some data, such as the pathological type, histological grade, and the expression of Ki-67, were incomplete. Secondly, the sample size was not large enough to reduce bias. Thirdly, the OS data was not mature, thus further follow-up was needed.
Conclusion
Overall, this study confirmed that pyrotinib was effective and safe among patients with HER2+ ABC in the real-world setting. Moreover, the ECOG-PS of 2–4, positive hormone receptor status, and presence of visceral metastasis were potential negative predictors of survival in HER2+ ABC.
Acknowledgments
The authors thank all the physicians and patients for the participation.
Funding Statement
This work was supported by the National Natural Science Foundation of China (Grant No.82072934 and No.81872156).
Abbreviations
HER2, human epidermal growth factor receptor 2; TKI, tyrosine kinase inhibitor; ABC, advanced breast cancer; EBC, early breast cancer; PFS, progression-free survival; mPFS, median PFS; OS, overall survival; mOS, median OS; ORR, objective response rate; CBR, clinical benefit rate; HR, hormone receptor; ER, estrogen receptor; PR, progesterone receptor; AEs, adverse events; BM, brain metastases; IHC, immunohistochemistry; FISH, fluorescence in-situ hybridization; RECIST, response evaluation criteria in solid tumors; NCI CTCAE, National Cancer Institute’s Common Terminology Criteria for Adverse Events; CIs, confidence intervals; SPSS, statistical package for social sciences; CSCO, Chinese Society of Clinical Oncology; FDA, Food and Drug Administration.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics Approval and Patient Consent
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethic Committee of the Second Hospital of Dalian Medical University. Informed consent was obtained from all individual participants included in the study.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.Choong GM, Cullen GD, O’Sullivan CC. Evolving standards of care and new challenges in the management of HER2-positive breast cancer. CA Cancer J Clin. 2020;70(5):355–374. doi: 10.3322/caac.21634 [DOI] [PubMed] [Google Scholar]
- 3.Xuhong J, Qi X, Zhang Y, et al. Mechanism, safety and efficacy of three tyrosine kinase inhibitors lapatinib, neratinib and pyrotinib in HER2-positive breast cancer. Am J Cancer Res. 2019;9(10):2103–2119. [PMC free article] [PubMed] [Google Scholar]
- 4.Vernieri C, Milano M, Brambilla M, et al. Resistance mechanisms to anti-HER2 therapies in HER2-positive breast cancer: current knowledge, new research directions and therapeutic perspectives. Crit Rev Oncol Hematol. 2019;139:53–66. doi: 10.1016/j.critrevonc.2019.05.001 [DOI] [PubMed] [Google Scholar]
- 5.Blair HA. Pyrotinib: first global approval. Drugs. 2018;78(16):1751–1755. doi: 10.1007/s40265-018-0997-0 [DOI] [PubMed] [Google Scholar]
- 6.Ma F, Ouyang Q, Li W, et al. Pyrotinib or lapatinib combined with capecitabine in HER2-positive metastatic breast cancer with prior taxanes, anthracyclines, and/or trastuzumab: a randomized, Phase II study. J Clin Oncol. 2019;37(29):2610–2619. doi: 10.1200/JCO.19.00108 [DOI] [PubMed] [Google Scholar]
- 7.Yan M, Bian L, Hu X, et al. Pyrotinib plus capecitabine for human epidermal factor receptor 2-positive metastatic breast cancer after trastuzumab and taxanes (PHENIX): a randomized, double-blind, placebo-controlled phase 3 study. Trans Breast Cancer Res. 2020;1:1–13. doi: 10.21037/tbcr-20-25 [DOI] [Google Scholar]
- 8.Xu B, Yan M, Ma F, et al. Pyrotinib plus capecitabine versus lapatinib plus capecitabine for the treatment of HER2-positive metastatic breast cancer (PHOEBE): a multicentre, open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 2021;22(3):351–360. doi: 10.1016/S1470-2045(20)30702-6 [DOI] [PubMed] [Google Scholar]
- 9.Yan M, Ouyang Q, Sun T, et al. Pyrotinib plus capecitabine for patients with human epidermal growth factor receptor 2-positive breast cancer and brain metastases (PERMEATE): a multicentre, single-arm, two-cohort, phase 2 trial. Lancet Oncol. 2022;23(3):353–361. doi: 10.1016/S1470-2045(21)00716-6 [DOI] [PubMed] [Google Scholar]
- 10.Wolff AC, Hammond MEH, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31(31):3997–4013. doi: 10.1200/JCO.2013.50.9984 [DOI] [PubMed] [Google Scholar]
- 11.de Melo Gagliato D, Jardim DLF, Marchesi MSP, et al. Mechanisms of resistance and sensitivity to anti-HER2 therapies in HER2+ breast cancer. Oncotarget. 2016;7(39):64431–64446. doi: 10.18632/oncotarget.7043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Swain SM, Kim S-B, Cortés J, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2013;14(6):461–471. doi: 10.1016/S1470-2045(13)70130-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu B, Li W, Zhang Q, et al. Pertuzumab, trastuzumab, and docetaxel for Chinese patients with previously untreated HER2-positive locally recurrent or metastatic breast cancer (PUFFIN): a Phase III, randomized, double-blind, placebo-controlled study. Breast Cancer Res Treat. 2020;182(3):689–697. doi: 10.1007/s10549-020-05728-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amiri-Kordestani L, Blumenthal GM, Xu QC, et al. FDA approval: ado-trastuzumab emtansine for the treatment of patients with HER2-positive metastatic breast cancer. Clin Cancer Res. 2014;20(17):4436–4441. doi: 10.1158/1078-0432.CCR-14-0012 [DOI] [PubMed] [Google Scholar]
- 15.Verma S, Miles D, Gianni L, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367(19):1783–1791. doi: 10.1056/NEJMoa1209124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saura C, Oliveira M, Feng YH, et al. Neratinib plus capecitabine versus lapatinib plus capecitabine in HER2-positive metastatic breast cancer previously treated with ≥ 2 HER2-directed regimens: Phase III NALA Trial. J Clin Oncol. 2020;38(27):3138–3149. doi: 10.1200/JCO.20.00147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang L, Wu X, Zhou J, et al. Pyrotinib in the treatment of women with HER2-positive advanced breast cancer: a multicenter, prospective, real-world study. Front Oncol. 2021;11:699323. doi: 10.3389/fonc.2021.699323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krop IE, Kim S-B, González-Martín A, et al. Trastuzumab emtansine versus treatment of physician’s choice for pretreated HER2-positive advanced breast cancer (TH3RESA): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15(7):689–699. doi: 10.1016/S1470-2045(14)70178-0 [DOI] [PubMed] [Google Scholar]
- 19.Leyland-Jones B. Human epidermal growth factor receptor 2-positive breast cancer and central nervous system metastases. J Clin Oncol. 2009;27(31):5278–5286. doi: 10.1200/JCO.2008.19.8481 [DOI] [PubMed] [Google Scholar]
- 20.Mehta AI, Brufsky AM, Sampson JH. Therapeutic approaches for HER2-positive brain metastases: circumventing the blood-brain barrier. Cancer Treat Rev. 2013;39(3):261–269. doi: 10.1016/j.ctrv.2012.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bachelot T, Romieu G, Campone M, et al. Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): a single-group phase 2 study. Lancet Oncol. 2013;14(1):64–71. doi: 10.1016/S1470-2045(12)70432-1 [DOI] [PubMed] [Google Scholar]
- 22.Freedman RA, Gelman RS, Anders CK, et al. TBCRC 022: a Phase II trial of neratinib and capecitabine for patients with human epidermal growth factor receptor 2-positive breast cancer and brain metastases. J Clin Oncol. 2019;37(13):1081–1089. doi: 10.1200/JCO.18.01511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murthy RK, Loi S, Okines A, et al. Tucatinib, trastuzumab, and capecitabine for HER2-positive metastatic breast cancer. N Engl J Med. 2020;382(7):597–609. doi: 10.1056/NEJMoa1914609 [DOI] [PubMed] [Google Scholar]
- 24.Lin NU, Borges V, Anders C, et al. Intracranial efficacy and survival with tucatinib plus trastuzumab and capecitabine for previously treated HER2-positive breast cancer with brain metastases in the HER2CLIMB trial. J Clin Oncol. 2020;38(23):2610–2619. doi: 10.1200/JCO.20.00775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anwar M, Chen Q, Ouyang D, et al. Pyrotinib treatment in patients with HER2-positive metastatic breast cancer and brain metastasis: exploratory final analysis of real-world, multicenter data. Clin Cancer Res. 2021;27(16):4634–4641. doi: 10.1158/1078-0432.CCR-21-0474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Konecny G, Pauletti G, Pegram M, et al. Quantitative association between HER-2/neu and steroid hormone receptors in hormone receptor-positive primary breast cancer. J Natl Cancer Inst. 2003;95(2):142–153. doi: 10.1093/jnci/95.2.142 [DOI] [PubMed] [Google Scholar]
- 27.Lal P, Tan LK, Chen B. Correlation of HER-2 status with estrogen and progesterone receptors and histologic features in 3655 invasive breast carcinomas. Am J Clin Pathol. 2005;123(4):541–546. doi: 10.1309/YMJ3A83TB39MRUT9 [DOI] [PubMed] [Google Scholar]
- 28.Schettini F, Buono G, Cardalesi C, et al. Hormone receptor/human epidermal growth factor receptor 2-positive breast cancer: where we are now and where we are going. Cancer Treat Rev. 2016;46:20–26. doi: 10.1016/j.ctrv.2016.03.012 [DOI] [PubMed] [Google Scholar]
- 29.Vici P, Pizzuti L, Natoli C, et al. Triple positive breast cancer: a distinct subtype? Cancer Treat Rev. 2015;41(2):69–76. doi: 10.1016/j.ctrv.2014.12.005 [DOI] [PubMed] [Google Scholar]
- 30.Montemurro F, Di Cosimo S, Arpino G. Human epidermal growth factor receptor 2 (HER2)-positive and hormone receptor-positive breast cancer: new insights into molecular interactions and clinical implications. Ann Oncol. 2013;24(11):2715–2724. doi: 10.1093/annonc/mdt287 [DOI] [PubMed] [Google Scholar]
- 31.Yan W, Wu X, Wang S, et al. Lobaplatin-based neoadjuvant chemotherapy for triple-negative breast cancer: a 5-year follow-up of a randomized, open-label, phase II trial. Ther Adv Med Oncol. 2022;14:1–10. doi: 10.1177/17588359221107111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Z, Wang C, Chen X, et al. Pathological response and predictive role of tumour-infiltrating lymphocytes in HER2-positive early breast cancer treated with neoadjuvant pyrotinib plus trastuzumab and chemotherapy (PANPHILA): a multicentre phase 2 trial. Eur J Cancer. 2022;165:157–168. doi: 10.1016/j.ejca.2022.01.022 [DOI] [PubMed] [Google Scholar]
- 33.Mao X, Lv P, Gong Y, et al. Pyrotinib-containing neoadjuvant therapy in patients with HER2-positive breast cancer: a multicenter retrospective analysis. Front Oncol. 2022;12:855512. doi: 10.3389/fonc.2022.855512 [DOI] [PMC free article] [PubMed] [Google Scholar]