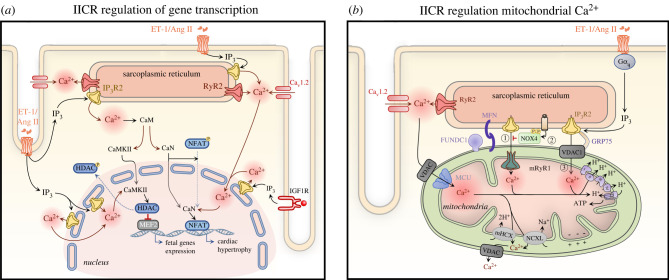
Figure 3.
InsP3-mediated signalling in regulation of gene expression and mitochondrial function. (a) Nuclear and cytosolic Ca2+ increases generated by Ca2+ release from InsP3Rs regulate gene expression underlying cardiomyocyte hypertrophic remodelling. Ca2+ released from InsP3Rs binds to calmodulin (CaM), which then activates CaM-dependent kinase II (CaMKII) and calcineurin (CaN). Activated CaMKII phosphorylates the inhibitory factor histone deacetylase (HDAC) and induces its export from the nucleus, resulting in MEF2 de-repression and induction of hypertrophic gene expression. Meanwhile, CaN dephosphorylates the nuclear factor of activated T-cells (NFAT) promoting its nuclear translocation and hypertrophic gene transcription. Ang II, angiotensin II; CaM, calmodulin; CaMKII, Ca2+/calmodulin-dependent protein kinase II; Cav1.2, α1C, subunit of voltage-gated L-type calcium channel; CaN - calcineurin; ET-1, endothelin-1; HDAC, histone deacetylase; IGF1R, insulin-like growth factor 1 receptor; IP3, inositol 1,4,5-trisphosphate; IP3R2, inositol trisphosphate receptor type 2; MEF2, myocyte enhancer factor-2; NFAT, nuclear factor of activated T cells, RyR2, ryanodine receptor type 2. (b) Mitochondrial Ca2+ uptake sites are closely localized to Ca2+ release sites at the junctional SR forming ‘hotspots’ with the help of tethers MFN and FUNDC1. Ca2+ released from the SR via RyRs is taken up via the voltage-gated anion channel (VDAC) associated with the mitochondrial Ca2+ uniporter (MCU) (1). In mitochondria, Ca2+ controls ATP production and apoptosis. Ca2+ is extruded from the mitochondria through Na+/Li+/Ca2+ exchanger (NCXL) and Ca2+/H+ exchanger (mHCX). Upon stimulation of Gαq by ET-1, Ang II or NE, InsP3 activates Ca2+ release from the SR leading to its uptake into the mitochondrial matrix through either VDAC (2) or mRyR1 (4). While Ca2+ transfer via VDAC1—GRP75—InsP3R results in induction of cell apoptosis (2), when taken up through mRyR1 it is associated with increased ATP production (4). To counterbalance InsP3-mediated mitochondrial Ca2+ overload during cellular stress, NOX4 augments the level of active phosphorylated AKT, which in turn phosphorylates and suppresses InsP3Rs thereby inhibiting Ca2+ flux from the SR to mitochondria (3). Akt, protein kinase B; Ang II, angiotensin II; ET-1, endothelin-1; FUNDC1, FUN14 domain-containing protein 1; GRP75, chaperone 75 kDa glucose-regulated protein; MCU, mitochondrial Ca2+ uniporter; MFN, mitofusin; mHCX, mitochondrial Ca2+/H+ exchanger; NCXL, Na+/Li+/Ca2+ exchanger; NE, norepinephrine; NOX4, NADPH oxidase 4; VDAC, voltage-gated anion channel. (Online version in colour.)