Abstract
Background:
It is unknown whether race- or insurance-based disparities in health care exist regarding baseline knee pain, knee function, complete meniscal tear, or articular cartilage damage in patients who undergo anterior cruciate ligament reconstruction (ACLR).
Hypothesis:
Black patients and patients with Medicaid evaluated for ACLR would have worse baseline knee pain, worse knee function, and greater odds of having a complete meniscal tear.
Study Design:
Cross-sectional study; Level of evidence, 3.
Methods:
A cohort of patients (N = 1463; 81% White, 14% Black, 5% Other race; median age, 22 years) who underwent ACLR between February 2015 and December 2018 was selected from an institutional database. Patients who underwent concomitant procedures and patients of undisclosed race or self-pay status were excluded. The associations of race with preoperative Knee injury and Osteoarthritis Outcome Score (KOOS) Pain subscale, KOOS Function subscale, and intraoperatively assessed complete meniscal tear (tear that extended through both the superior and the inferior meniscal surfaces) were determined via multivariate modeling with adjustment for age, sex, insurance status, years of education, smoking status, body mass index (BMI), meniscal tear location, and Veterans RAND 12-Item Health Survey Mental Component Score (VR-12 MCS).
Results:
The 3 factors most strongly associated with worse KOOS Pain and KOOS Function were lower VR-12 MCS score, increased BMI, and increased age. Except for age, the other two factors had an unequal distribution between Black and White patients. Univariate analysis demonstrated equal baseline median KOOS Pain scores (Black, 72.2; White, 72.2) and KOOS Function scores (Black, 68.2; White, 68.2). After adjusting for confounding variables, there was no significant difference between Black and White patients in KOOS Pain, KOOS Function, or complete meniscal tears. Insurance status was not a significant predictor of KOOS Pain, KOOS Function, or complete meniscal tear.
Conclusion:
There were clinically significant differences between Black and White patients evaluated for ACLR. After accounting for confounding factors, no difference was observed between Black and White patients in knee pain, knee function, or complete meniscal tear. Insurance was not a clinically significant predictor of knee pain, knee function, or complete meniscal tear.
Keywords: racial disparities, anterior cruciate ligament reconstruction, insurance status, meniscal tear, patient-reported outcome
Health care disparities have been long recognized in the United States. Patients of a certain race, ethnicity, socioeconomic status, or insurance status have been found to be at an increased risk of poorer outcomes. 16,21,23,27,39,49 Racial disparities are well documented in all medical specialties. †† The reasons for racial disparities in surgical patients are considered multifactorial 28,32 and consist of issues including but not limited to insurance provider, patient characteristics, and socioeconomic status. 1,32
Much of the orthopaedic literature has focused on disparities in joint arthroplasty and spine surgery. ‡‡ Several studies have reported decreased joint arthroplasty utilization in Black patients compared with White patients. 6,43 Using a Medicare database (MedPAR), Singh et al 42 found persistent racial disparities in joint arthroplasty usage and outcomes, with Black patients having significantly lower utilization rates and poorer outcomes over the 18-year study period. Similar disparities have been reported based on insurance status. 21,25,39,49 Schoenfeld et al 39 analyzed the outcomes of patients with spinal trauma in a national database and found that patients lacking insurance were at an increased risk of mortality compared with those with private commercial insurance. Patients who lacked insurance also had a significantly decreased number of hospital days, days in the intensive care unit, and time on a ventilator.
Previous studies in arthroplasty and spine surgery populations have relied on national databases that are capable of providing information on outcomes and health care disparity. No such standardized database exists for the subspecialty of sports medicine. Furthermore, studies focusing on health care disparities in arthroplasty and the spine population do not necessarily apply to sports medicine populations, specifically those undergoing anterior cruciate ligament (ACL) reconstruction (ACLR). ACLR is one of the most commonly performed sports medicine procedures in the United States, with an estimated annual incidence ranging from 60,000 to 175,000, and previous reviews have shown the incidence to be increasing. 8,22,24,38 Despite the increased attention to health care disparities over the past 2 decades and the increased incidence of ACLR, there remain no studies specifically evaluating the existence of health care disparities in the ACLR population.
The objective of this study was to evaluate the possibility of health care disparities in validated patient-reported outcome measures (PROMs) for pain and function and meniscal tears in patients undergoing ACLR. We hypothesized that Black patients and patients with Medicaid evaluated for ACLR would have worse knee pain, worse knee function, and more meniscal tears.
Methods
Participants
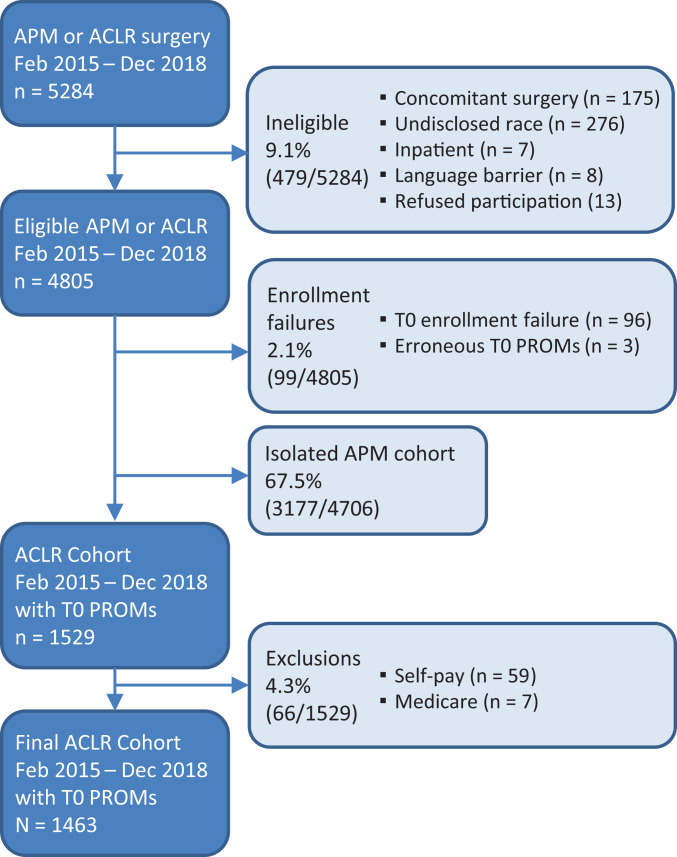
This study was approved by our institutional review board. All patients undergoing elective sports medicine and arthroscopic procedures at the Cleveland Clinic were prospectively enrolled in the Outcomes Measurement Evaluation (OME) cohort. 29 As a part of standard of care and alteration of consent OME is a secure data collection system that has been developed at the Cleveland Clinic to prospectively capture patient characteristics (height, weight, years of education, smoking status), joint-specific and whole-body PROMs, quality of life scores, physical examination findings, and intraoperative surgical findings. The current study population was identified from an initial cohort of 5284 patients who underwent arthroscopic partial meniscectomy or ACLR between February 2015 and December 2018. Exclusion criteria were (1) concomitant complex surgery (eg, multiligament knee injury, patellofemoral surgery, quadriceps tendon repair), (2) inpatient stay at time of surgery, (3) “self-pay” insurance, (4) language barrier, (5) refusal to participate in OME, (6) lack of completion of patient PROMs or intraoperative surgical findings, and (7) race not listed in the electronic medical record (EMR). After application of all exclusion criteria, a total of 1463 patients were included in the current study analysis (Figure 1).
Figure 1.
The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) diagram. ACLR, anterior cruciate ligament reconstruction; APM, arthroscopic partial meniscectomy; PROM, patient-reported outcome measure; T0, time zero (baseline).
Data Collection
After identification of all eligible patients, data from OME were then exported to a secure Research Electronic Data Capture (REDCap, Vanderbilt University) database. 15 Patient age at time of surgery, sex, body mass index (BMI), smoking status, PROM scores, and intraoperative findings were all collected prospectively as part of OME. Race and insurance status were obtained via an automated extract from the EMR. Race entries available in the EMR included White, Black, Asian, multiracial, Native American, Other, and not listed; these entries are based on patient self-identification on intake forms when first establishing care within the hospital system. All races not reported as Black or White were classified as Other for the purpose of this study.
All PROMs were collected before surgery. Preoperative knee pain was assessed using the Knee injury and Osteoarthritis Outcome Score (KOOS) Pain subscale (KOOS Pain). 36 Preoperative patient-reported knee function was assessed using the KOOS Physical Function short form. 30 This form, often abbreviated in the literature as KOOS PS, is not one of the original KOOS subscales. It is shorter, with fewer questions to reduce responder burnout. In the current article, we refer to this scale as KOOS Function. Finally, mental health was assessed using the Veterans RAND 12-Item Health Survey Mental Component Score (VR-12 MCS). 41
Intraoperative findings were captured by the surgeon and entered directly by the surgeon into OME. Concomitant meniscal pathology was managed with partial meniscectomy or repair of the medial and/or lateral meniscus. A complete tear was defined as a tear that extended through both the superior and the inferior meniscal surfaces or a tear extending from the central meniscus to the meniscal periphery. The surgeon noted the location of the complete tear as medial, lateral, or both menisci. Tear involvement of the meniscus root was also noted.
Statistical Analysis
A power analysis was initially carried out to verify the ability of this study to detect differences in the groups studied. The generally accepted minimal clinically important difference (MCID) of 8 points 15 was used for KOOS Pain and KOOS Function, and a true odds ratio of 1.5 was used for complete meniscal tear in the power analysis. The study was adequately powered (>80% power at alpha = .05) for comparing outcomes between Black and White patients but was underpowered for comparing patients of Other race (n = 85; 3% of the study sample) with Black or White patients.
Descriptive statistics were generated for the entire sample population. Normal variables were summarized using mean and standard deviation, and nonnormal variables were summarized using median and interquartile range. The normality of continuous measures was determined using the Shapiro-Wilk test. Categorical variables were summarized using frequency. Univariate relationships with race (White, Black, Other) were analyzed using analysis of variance (normal continuous variables), the Kruskal-Wallis test (nonnormal continuous variables), and the Pearson chi-square test (categorical variables). For comparisons between the groups, we used Tukey adjustments when the row variable was normally distributed and the Benjamini-Hochberg method otherwise.
Multivariate linear regression was used to identify the risk factors associated with KOOS Pain and KOOS Function. A multivariate proportional odds regression model was used to identify risk factors associated with complete meniscal tears. Linear regression assumptions (independent and normally distributed, with mean = 0 and constant variance) were checked and verified via residual graphs. The proportional odds assumption was checked graphically as well. All multivariate models included race, sex, insurance type, BMI, years of education, smoking status, baseline VR-12 MCS score, and meniscal tear characteristics. The interactions of race × insurance and patient age × years of education were tested in each model, in which a significant interaction indicated that the magnitude effect of 1 variable on the outcome depended on the second variable and vice versa. The race × insurance interaction terms were kept in all models, but the age × years of education interaction term was excluded if it was not significant. To measure the relative importance of individual variables in the regression models, we created Akaike information criterion (AIC) importance plots. These plots illustrate the magnitude change in the AIC value, a measure of model goodness of fit, when the individual variable was removed from the statistical model. Finally, the overall quality of the regression models for KOOS Pain, KOOS Function, and meniscal tears were assessed using a validated, index-adjusted R 2 and concordance index. P values <.05 were considered significant. Analysis was done in R Software (Version 3.5).
Results
Descriptive Statistics and Univariate Comparisons Between Races
There were 1190 White patients (81%), 201 Black patients (14%), and 72 patients (5%) considered Other (Table 1). There were multiple clinically and statistically significant differences in the distribution of sociodemographic variables between Black and White races (Table 1). White patients had a lower mean BMI (White, 26.9 vs Black, 28.4; P = .002). White patients were more likely to have a commercial insurance plan (White, 93.0% vs Black, 57.7%; P < .001). White patients were less likely to be current smokers (White, 6.4% vs Black, 21.4%; P < .001). There was a statistically significant but likely clinically nonsignificant difference in median years of education (White, 13 years vs Black, 12 years; P < .001).
Table 1.
Descriptive Statistics by Race a
| Race | P | |||||
|---|---|---|---|---|---|---|
| Variable | White, n = 1190 | Black, n = 201 | Other, n = 72 | White vs Black | White vs Other | Black vs Other |
| Age at surgery, y | 22.0 [17.0-36.0] | 21.0 [17.0-31.0] | 24.0 [17.0-32.0] | .313 | .781 | .727 |
| Sex | .390 | .332 | .390 | |||
| Male | 620 (52.1) | 112 (55.7) | 45 (62.5) | |||
| Female | 570 (47.9) | 89 (44.3) | 27 (37.5) | |||
| Insurance type | <.001 | .005 | <.001 | |||
| Commercial | 1107 (93.0) | 116 (57.7) | 60 (83.3) | |||
| Medicaid | 83 (7.0) | 85 (42.3) | 12 (16.7) | |||
| BMI | 26.9 ± 5.61 | 28.4 ± 7.37 | 26.4 ± 4.86 | .002 | .763 | .031 |
| Years of education | 13.0 [11.0-16.0] | 12.0 [11.0-14.0] | 14.0 [11.0-16.0] | <.001 | .167 | .001 |
| Years of education by group | .198 | .336 | .198 | |||
| ≥12 | 785 (66.0) | 121 (60.2) | 52 (72.2) | |||
| <12 | 405 (34.0) | 80 (39.8) | 20 (27.8) | |||
| Smoking status | <.001 | .755 | .057 | |||
| Never | 955 (80.3) | 141 (70.1) | 57 (79.2) | |||
| Quit | 159 (13.4) | 17 (8.5) | 9 (12.5) | |||
| Current | 76 (6.4) | 43 (21.4) | 6 (8.33) | |||
| Baseline VR-12 MCS | 56.3 [48.1-61.3] | 53.3 [43.1-59.4] | 55.4 [47.3-60.3] | .001 | .614 | .160 |
| Meniscus root tear | >.999 | >.999 | >.999 | |||
| No | 1151 (96.7) | 195 (97.0) | 70 (97.2) | |||
| Yes | 39 (3.3) | 6 (3.0) | 2 (2.8) | |||
| Tear location | .004 | .866 | .405 | |||
| None | 606 (50.9) | 81 (40.3) | 36 (50.0) | |||
| Medial | 205 (17.2) | 32 (15.9) | 14 (19.4) | |||
| Lateral | 252 (21.2) | 49 (24.4) | 13 (18.1) | |||
| Both | 127 (10.7) | 39 (19.4) | 9 (12.5) | |||
| Baseline KOOS | ||||||
| Pain | 72.2 [58.3-83.3] | 72.2 [50.0-83.3] | 72.2 [61.1-86.1] | .412 | .563 | .412 |
| Function | 68.2 [61.4-78.0] | 68.2 [58.0-78.0] | 69.2 [58.0-78.8] | .151 | .647 | .647 |
| Extent of OA | .248 | .575 | .248 | |||
| Normal or G1 | 809 (68.0) | 123 (61.2) | 53 (73.6) | |||
| G2 | 184 (15.5) | 39 (19.4) | 10 (13.9) | |||
| G3, G4, or OCD | 197 (16.6) | 39 (19.4) | 9 (12.5) | |||
a Data are reported as median [interquartile range], n (%), or mean ± SD. Boldface P values indicate a statistically significant difference between groups compared (P < .05). BMI, body mass index; G1, G2, G3, G4, grades 1-4; KOOS, Knee injury and Osteoarthritis Outcome Score; OA, osteoarthritis; OCD, osteochondral defect; VR-12 MCS, Veterans RAND 12-Item Health Survey Mental Component Score.
Regarding preoperative PROMs, equivalent baseline scores were observed between Black and White patients in median KOOS Pain (Black, 72.2 vs White, 72.2; P = .412) and KOOS Function (Black, 68.2 vs White, 68.2; P = .151). There was a statistically significant difference in median VR-12 MCS scores (Black, 53.3 vs White, 56.3; P = .001). Intraoperative findings showed differences by race in the presence and location of complete meniscal tears (P = .004) but no difference in meniscus root involvement (P > .999).
Comparisons of patients of Other race with Black and White patients are provided (Table 1); because of the small size of the Other group, these statistical comparisons were underpowered and should be interpreted with caution.
Independent Associations With KOOS Pain, KOOS Function, and Articular Cartilage Grade
Evaluating race (Black vs White) as a risk factor for PROMs or intra-articular meniscal injuries required a multivariable analysis. This analysis was adjusted for other risk factors either known to be associated or hypothesized to be associated with a specific PROM or meniscal tear/articular injury. These risk factors, adjusted for confounding variables, are shown in Table 2.
Table 2.
Linear Regression Model Results: KOOS Pain a
| Variable | Estimate (95% CI) b | P |
|---|---|---|
| Age | –0.41 (–0.5 to –0.32) | <.001 |
| Sex, female (vs male) | –2.56 (–4.27 to –0.84) | .004 |
| Race | ||
| Black (vs White) | 0.7 (–2.47 to 3.86) | .666 |
| Other (vs White) | 0.48 (–3.77 to 4.74) | .823 |
| BMI | –0.54 (–0.69 to –0.39) | <.001 |
| Years of education | 0.56 (0.27 to 0.84) | <.001 |
| Smoking status | ||
| Quit (vs never) | –0.69 (–3.35 to 1.97) | .611 |
| Current (vs never) | –7.92 (–11.15 to –4.7) | <.001 |
| Baseline VR-12 MCS | 0.37 (0.29 to 0.46) | <.001 |
| Insurance, Medicaid (vs commercial) | –6.13 (–9.87 to –2.38) | .001 |
| Tear location | ||
| Medial (vs no tear) | –0.19 (–2.55 to 2.17) | .873 |
| Lateral (vs no tear) | 1.48 (–0.7 to 3.67) | .183 |
| Both (vs no tear) | –0.47 (–3.19 to 2.25) | .733 |
| Race × insurance | ||
| Black × Medicaid | 2.17 (–3.69 to 8.03) | .468 |
| Other × Medicaid | 1.1 (–9.72 to 11.91) | .843 |
a Boldface P values indicate a statistically significant difference between groups compared (P < .05). BMI, body mass index; KOOS, Knee injury and Osteoarthritis Outcome Score; VR-12 MCS, Veterans RAND 12-Item Health Survey Mental Component Score.
b This value represents the estimated change in KOOS Pain. As an example, the estimated change in KOOS Pain per 1-year increase in patient age was –0.41 points. The estimated difference in KOOS Pain among female versus male patients was –2.56 points.
There was no difference between Black and White patients in KOOS Pain scores after adjusting for relevant confounding variables (Table 2). Insurance type showed a difference in KOOS Pain score, with patients with Medicaid expected to have a KOOS Pain score worse than that for patients with commercial insurance by a mean of 6.13 points (95% CI, 9.87-2.38; P = .001). The magnitude of difference was not dependent on race. The main drivers of KOOS Pain in order of importance were age, baseline mental health (VR-12 MCS), BMI, smoking status, and years of education (Figure 2). After accounting for confounding variables, neither race nor insurance showed a difference in KOOS Function (Table 3). The main drivers of KOOS Function in order of importance were baseline mental health (VR-12 MCS), age, BMI, smoking status, and years of education (Figure 3). Four out of five most important drivers (except age) of KOOS Pain and KOOS Function in the multivariate analyses were unequally distributed between Black and White patients in the univariate analysis (Table 1).
Figure 2.
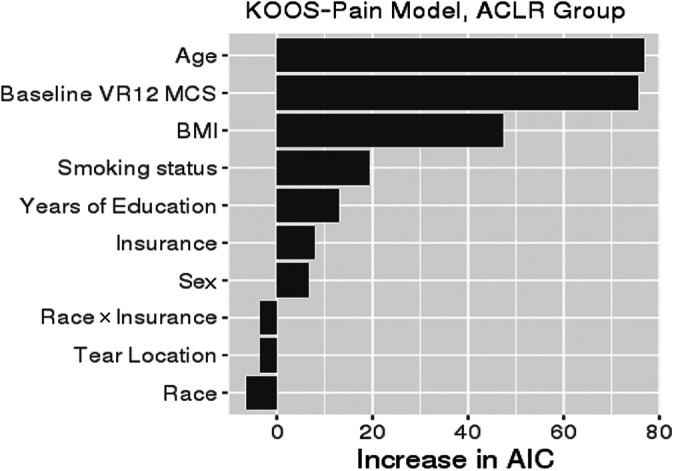
Variable importance plot for KOOS Pain. The relative importance of each variable in explaining KOOS Pain was ranked according to the increase in AIC upon removal from the full model. When race or insurance was removed, the race × insurance interaction was removed as well. ACLR, anterior cruciate ligament reconstruction; AIC, Akaike information criterion; BMI, body mass index; KOOS, Knee injury and Osteoarthritis Outcome Score; VR-12 MCS, Veterans RAND 12-Item Health Survey Mental Component Score.
Table 3.
Linear Regression Model Results: KOOS Function a
| Variable | Estimate (95% CI) b | P |
|---|---|---|
| Age | –0.29 (–0.36 to –0.22) | <.001 |
| Sex, female (vs male) | –2.19 (–3.58 to –0.81) | .002 |
| Race | ||
| Black (vs White) | 0.29 (–2.28 to 2.85) | .826 |
| Other (vs White) | –1.69 (–5.13 to 1.76) | .337 |
| BMI | –0.4 (–0.52 to –0.28) | <.001 |
| Years of education | 0.4 (0.18 to 0.63) | .001 |
| Smoking status | ||
| Quit (vs never) | 0.29 (–1.87 to 2.45) | .792 |
| Current (vs never) | –7.11 (–9.72 to –4.5) | <.001 |
| Baseline VR-12 MCS | 0.28 (0.22 to 0.35) | <.001 |
| Insurance, Medicaid (vs commercial) | –2.86 (–5.89 to 0.18) | .065 |
| Tear location | ||
| Medial (vs no tear) | 0.44 (–1.47 to 2.35) | .652 |
| Lateral (vs no tear) | 1.01 (–0.75 to 2.78) | .262 |
| Both (vs no tear) | 1.28 (–0.92 to 3.48) | .255 |
| Race × insurance | ||
| Black × Medicaid | –0.48 (–5.22 to 4.27) | .844 |
| Other × Medicaid | 0.3 (–8.46 to 9.06) | .946 |
a Boldface P values indicate statistical significance (P < .05). BMI, body mass index; KOOS, Knee injury and Osteoarthritis Outcome Score; VR-12 MCS, Veterans RAND 12-Item Health Survey Mental Component Score.
b This value represents the estimated change in KOOS Function. As an example, the estimated change in KOOS Function per 1-year increase in patient age is –0.29 points. The estimated difference in KOOS Function among female versus male patients is –2.19 points.
Figure 3.
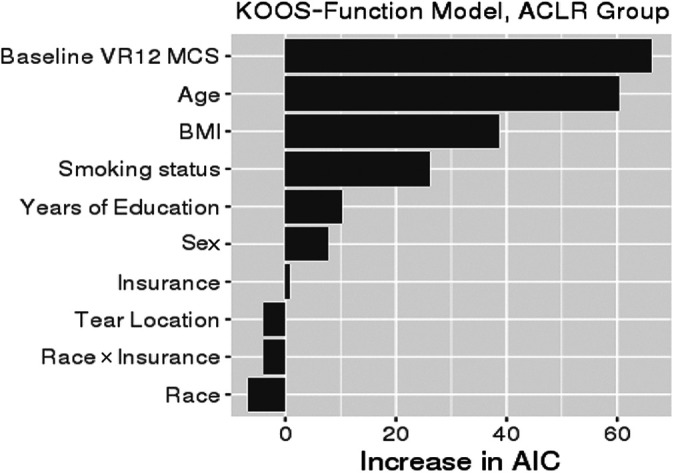
Variable importance plot for KOOS Function. The relative importance of each variable in explaining KOOS Function was ranked according to the increase in AIC upon removal from the full model. When race or insurance was removed, the race × insurance interaction was removed as well. ACLR, anterior cruciate ligament reconstruction; AIC, Akaike information criterion; BMI, body mass index; KOOS, Knee injury and Osteoarthritis Outcome Score; VR-12 MCS, Veterans RAND 12-Item Health Survey Mental Component Score.
After adjusting for confounding variables, the risk of a complete meniscal tear was not significantly different between Black and White patients. Neither race nor insurance was a significant predictor of complete meniscal tear. The 2 most important drivers of complete meniscal tear were sex and years of education (Figure 4). The most important driver of complete meniscal tear in the multivariate analysis was unequally distributed between Black and White patients in the univariate analysis.
Figure 4.
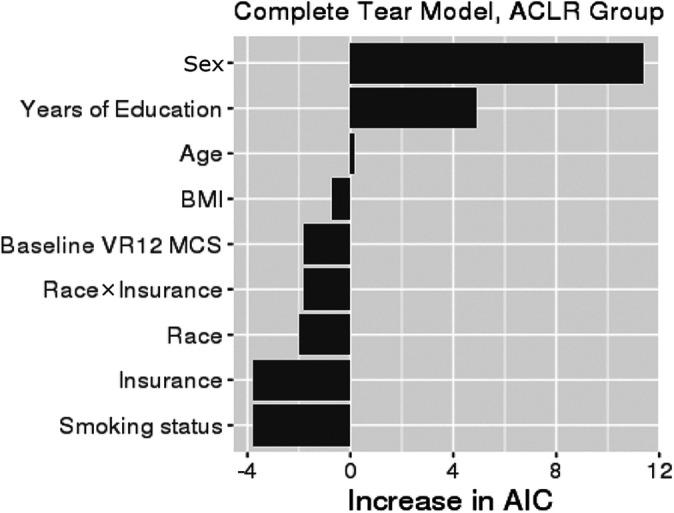
Variable importance plot for complete meniscal tear. The relative importance of each variable in explaining complete meniscal tear was ranked according to the increase in AIC upon removal from the full model. When race or insurance was removed, the race × insurance interaction was removed as well. ACLR, anterior cruciate ligament reconstruction; AIC, Akaike information criterion; BMI, body mass index; VR-12 MCS, Veterans RAND 12-Item Health Survey Mental Component Score.
The results of the multivariate analyses are summarized in Table 5. Because of the small size of the Other group, comparisons of Other race patients with Black and White patients in the multivariate analyses were relatively underpowered and should be interpreted with caution.
Table 5.
Summary of Significant Variables in Multivariate Analysis a
| Variable | KOOS Pain | KOOS Function | Complete Meniscal Tear |
|---|---|---|---|
| Age | X | X | — |
| Sex, female (vs male) | X | X | X |
| Race | — | — | — |
| Black (vs White) | — | — | — |
| Other (vs White) | — | — | — |
| BMI | X | X | — |
| Years of education | X | X | X |
| Smoking status | — | — | — |
| Quit (vs never) | — | — | — |
| Current (vs never) | X | X | — |
| Baseline VR-12 MCS | X | X | — |
| Insurance | — | — | — |
| Medicare (vs commercial) | X | — | — |
| Medicaid (vs commercial) | — | — | — |
| Meniscal tear, yes (vs no) | — | — | — |
| Tear location | — | — | — |
| Medial (vs no tear) | — | — | — |
| Lateral (vs no tear) | — | — | — |
| Both (vs no tear) | — | — | — |
| Race × insurance | — | — | — |
| Black × Medicare | — | — | — |
| Other × Medicare | — | — | — |
| Black × Medicaid | — | — | — |
| Other × Medicaid | — | — | — |
| Patient age × years of education | — | — | — |
a X indicates significant; — indicates not significant. BMI, body mass index; KOOS, Knee injury and Osteoarthritis Outcome Score; VR-12 MCS, Veterans RAND 12-Item Health Survey Mental Component Score.
Table 4.
Proportional Odds Regression Model Results: Complete Meniscal Tear a
| Variable | Odds Ratio (95% CI) b | P |
|---|---|---|
| Age | 1.01 (1-1.02) | .143 |
| Sex, female (vs male) | 0.67 (0.55-0.83) | <.001 |
| Race | ||
| Black (vs White) | 1.22 (0.83-1.81) | .313 |
| Other (vs White) | 1.15 (0.68-1.94) | .607 |
| BMI | 1.01 (0.99-1.03) | .262 |
| Years of education | 0.95 (0.92-0.99) | .009 |
| Smoking status | ||
| Quit (vs never) | 0.93 (0.67-1.29) | .669 |
| Current (vs never) | 1 (0.67-1.5) | .982 |
| Baseline VR-12 MCS | 1 (0.99-1.01) | .660 |
| Insurance, Medicaid (vs commercial) | 0.94 (0.59-1.5) | .797 |
| Race × insurance | ||
| Black × Medicaid | 1.49 (0.71-3.12) | .291 |
| Other × Medicaid | 0.59 (0.15-2.25) | .436 |
a Boldface P values indicate statistical significance (P < .05). BMI, body mass index; VR-12 MCS, Veterans RAND 12-Item Health Survey Mental Component Score.
b This value represents the increased odds of a complete meniscal tear. As an example, the odds of a complete meniscal tear increases 1.01-fold per 1-year increase in patient age.
Discussion
The principal findings of our study of >1400 patients demonstrated that although characteristic differences exist between Black and White patients, after adjusting for these factors, no racial disparity exists with regard to knee pain, knee function, or the presence of complete meniscal tear in Black and White patients undergoing ACLR. After adjusting for confounding variables, insurance type was a predictor of baseline knee pain, with patients with Medicaid demonstrating significantly worse pain than patients with commercial insurance. This difference did not persist with regard to knee function or complete meniscal tears. This study is the first to evaluate health care disparities in ACLR. The findings of this study are contrary to previously documented reports in joint arthroplasty and spine surgery. §§
The disparities expected for Black patients in knee pain, knee function, and complete meniscal tear and/or articular cartilage damage were absent in the present study. This is an unexpected finding considering previously reported outcomes. Even though ACL injuries represent a traumatic event, the surgery remains elective in nature. The median age in the present study was 22 years for White patients and 21 years for Black patients, which would include a significant population of patients who were injured in recreational events that do not have on-site medical provider coverage. In these cases, the desire to seek care would be elective depending on injury evaluation.
Racial disparities have been identified in elective arthroplasty and spine surgery as well as trauma surgery. Traditionally, studies have used utilization and complication rates to highlight disparities between Black and White patients. 34,39,40,42 –44,48 It is believed that the evaluation of Black patients is delayed because of a variety of personal and institutional factors. This would suggest that Black patients are evaluated with worse underlying pathology. 6,33,42 However, the evaluation of patients undergoing ACLR is different from that of patients undergoing elective joint arthroplasty or spine surgery. ACL injuries typically occur as an identifiable incident with associated pain and effusion and a known mechanism of injury. ACL injuries are also debilitating, and patients possibly are unable to ignore them or delay their treatment as easily as pathology involved in simple arthroscopy or other orthopaedic procedures. This may lead patients to seek care sooner rather than later. Furthermore, the institution has created a standardized process meant to streamline treatment of patients with ACL injuries. This system is built on a foundation of community outreach and sports coverage that is not present in previously studied populations. This may explain access for high school and collegiate athletes across backgrounds, but it does not fully explain the outcome as it relates to recreational athletes. Ultimately, patient evaluation is based on the injury and not patient- or provider-related biases.
Similarly, there was no difference in knee function and complete meniscal tears based on insurance status. After adjusting for multiple factors, a modest but significant disparity in KOOS Pain scores was observed between patients with Medicaid and commercial insurance. Based on the linear regression analysis, patients with Medicaid were expected to have a KOOS Pain score worse than that of patients with commercial insurance by about 6.13 points. Although this difference was statistically significant, it is likely clinically nonsignificant. The MCID for the KOOS Pain score has previously been reported at approximately 8 points. 2,3,24,35 However, care should be taken when applying strict MCID values. Currently, there is no consensus regarding MCID values. Furthermore, MCID values can be influenced by calculation method, follow rate, follow timing, and so forth. MCID cannot be strictly applied across various studies. We included this in this study as a general point of reference, but we did not specially calculate or address MCID, as this was a baseline analysis. 17
Insurance status was not a significant driver of knee function or complete meniscal tears. Martin et al 25 found that patients undergoing joint arthroplasty with Medicaid were evaluated with worse preoperative pain and function scores than similar patients with private insurance or Medicare. This continues to highlight the differences between joint arthroplasty and ACLR populations. The previously outlined manner in which these populations are evaluated is one explanation for the opposing outcomes. Most previous studies have specifically highlighted a lack of insurance as an important predictor of outcome. 37,39 All patients in the present study were insured, either via commercial insurance or a government-sponsored program.
There were several variables associated with KOOS values. Baseline VR-12 MCS was one of the strongest predictors of KOOS values. Mental health has demonstrated a strong association with knee pain, which is demonstrated in the baseline KOOS Pain and KOOS Function values. 31 The association of psychological and mental health factors with outcomes after ACLR has been well documented in previous reviews. 11,12 Higher BMI was another strong predictor for poorer KOOS values at baseline. This association has been demonstrated in previous reports. 18,19,45,46 However, the clinically relevant association of BMI has been questioned by Spindler et al, 45,46 who found that individual differences in outcomes were below a clinically meaningful difference. 45,46 Current smoking status and years of education had a modest but significant association with KOOS values. Recent literature has suggested that smoking has an independent negative effect on knee ligament surgery. 10,45,46,49 Years of education can act as a surrogate for health care literacy. This has been demonstrated in previous studies in which years of education had a positive association with health across a variety of conditions. 9 All these significant variables were statistically significantly different between White and Black patients, with Black patients having lower baseline mental health scores, higher baseline BMI, higher rates of current smokers, and fewer years of education (Table 1).
Limitations and Strengths
There are several important limitations to this study. The first is that this study was performed at a single large hospital system in the midwestern United States. It is possible that patients in this geographic region are not representative of the national patient population. Furthermore, care paths for patients with ACL injuries may not be present in all institutions. Because of the limited sample size of non-Black and non-White patients, we were only able to evaluate White-Black disparities with adequate statistical power. All patients in the sample were insured with commercial insurance or Medicaid. Therefore, it was not possible to completely evaluate the significance of insurance status.
There are several strengths to this study. This work was able to account for a number of confounders using logistic regression techniques, something that is lacking in prior studies. This study benefits from the use of a large prospective database that captures sociodemographic characteristics and outcomes in a prospective manner (OME). This is a unique form of data capture, as most standardized surgical registries do not record PROMs. We believe that this allows us to more accurately evaluate the effect an orthopaedic condition has on individual patients. Finally, to our knowledge this is the first study to evaluate health care disparities in patients undergoing ACLR. The findings support the belief that previously reported health care disparities cannot be generalized across specialties and procedures. Further research is needed to confirm the present findings as well as identify whether racial disparities exist in other areas of sports medicine. Furthermore, interaction with minority communities will be necessary to identify the cause(s) of racial disparities observed in the other areas of orthopaedics.
Conclusion
There were clinically significant differences between Black and White patients evaluated for ACLR. After accounting for other confounding factors (risk factors related to outcome of interest), no clinically relevant difference was observed between Black and White patients or patients with Medicaid and commercial insurance in knee pain, knee function, or complete meniscal tear.
Acknowledgment
The authors thank the Cleveland Clinic orthopaedic patients, staff, and research personnel whose efforts related to regulatory requirements, data collection, patient follow-up, data quality control, analyses, and manuscript preparation have made this consortium successful. Thanks also to the following surgeons for contributing cases: Thomas E. Anderson, MD, Jack T. Andrish, MD, Alan W. Davis, MD, Robert J. Hampton, MD, Michael W. Kolczun, MD, David H. Krahe, MD, Richard R. Masin, MD, Andrew J. Matko, MD, Victor A. Nemeth, MD, Bradley A. Pierce, MD, and Joseph B. Scarcella, MD. Thank you to William Messner, MS, for the initial statistical analysis. Thank you to Cailin Conner, MA, for editorial management.
Footnotes
All authors are listed in the Authors section at the end of the article.
AUTHORS: Lutul D. Farrow, MD; Michael J. Scarcella, MD (Orthopaedic and Rheumatologic Institute, Cleveland Clinic, Garfield Heights, Ohio, USA); Christa L. Wentt, MD (Department of Orthopaedic Surgery, Howard University Hospital, Washington DC, USA); Morgan H. Jones, MD, MPH (Division of Sports Medicine, Department of Orthopedic Surgery, Brigham and Women’s Hospital, Boston, Massachusetts, USA); Cleveland Clinic OME Sports Medicine group: Isaac Briskin, MS (Department of Quantitative Health Services, Cleveland Clinic, Cleveland, Ohio, USA) and Brian M. Leo, MD, Brett W. McCoy, MD, Anthony A. Miniaci, MD, Richard D. Parker, MD, James T. Rosneck, MD, Frank M. Sabo, MD, Paul M. Saluan, MD, and Alfred Serna, MD (Orthopaedic and Rheumatologic Institute, Cleveland Clinic, Cleveland, Ohio, USA); Kurt P. Spindler, MD (Department of Orthopaedic Surgery, Cleveland Clinic Florida Region, Weston, Florida, USA); and Kim L. Stearns, MD, Gregory J. Strnad, MS, and James S. Williams, MD (Orthopaedic and Rheumatologic Institute, Cleveland Clinic, Cleveland, Ohio, USA).
Final revision submitted April 11, 2022; accepted May 17, 2022.
One or more of the authors has declared the following potential conflict of interest or source of funding: Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health (award K23AR066133), which supported a portion of the professional effort for M.H.J. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. L.D.F. has received hospitality payments from DJO. M.J.S. has received education payments from Smith & Nephew and hospitality payments from DJO. B.M.L. has received education payments from Southern Edge Orthopaedics and Zimmer Biomet and consulting fees from Cayenne Medical and Zimmer Biomet. A.A.M. has received education payments from Rock Medical; consulting fees from Amniox Medical, Anika Therapeutics, Arthrosurface, Linvatec, Stryker, and Trice; speaking fees from Trice; royalties from Wolters Kluwer and Zimmer Biomet; hospitality payments from Arthrex, DJO, and Smith & Nephew; and honoraria from Arthrosurface and has stock/stock options in Anika Therapeutics, Arthrosurface, and Trice. R.D.P. has received hospitality payments from Musculoskeletal Transplant Foundation and Smith & Nephew and royalties from Zimmer Biomet. J.T.R. has received consulting fees from Smith & Nephew. F.M.S. has received education payments from Arthrex and hospitality payments from Stryker. P.M.S. has received education payments from Rock Medical; consulting fees from Arthrex, DePuy, and DJO; nonconsulting fees from Arthrex; and hospitality payments from Musculoskeletal Transplant Foundation. A.S. has received education payments from Arthrex. K.P.S. has received research support from DJO and Smith & Nephew and consulting fees from Flexion Therapeutics, National Football League, and Novopeds. K.L.S. has received education payments from Arthrex and Biomet Orthopedics; consulting fees from Heron Therapeutics; and hospitality payments from Stryker Corporation, Horizon Pharma, Musculoskeletal Transplant Foundation, Biomet Orthopedics, and Ramsay Medical. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval for this study was obtained from Cleveland Clinic (reference No. 15-702).
References
- 1. Balsa AI, Seiler N, McGuire TG, Bloche MG. Clinical uncertainty and healthcare disparities. Am J Law Med. 2003;29(2-3):203–219. [PubMed] [Google Scholar]
- 2. Berliner JL, Brodke DJ, Chan V, SooHoo NF, Bozic KJ. John Charnley Award: preoperative patient-reported outcome measures predict clinically meaningful improvement in function after THA. Clin Orthop Relat Res. 2016;474(2):321–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Berliner JL, Brodke DJ, Chan V, SooHoo NF, Bozic KJ. Can preoperative patient-reported outcome measures be used to predict meaningful improvement in function after TKA? Clin Orthop Relat Res. 2017;475(1):149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cai X, Cram P, Vaughan-Sarrazin M. Are African American patients more likely to receive a total knee arthroplasty in a low-quality hospital? Clin Orthop Relat Res. 2012;470(4):1185–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Carbone S, Gumina S, Arceri V, Campagna V, Fagnani C, Postacchini F. The impact of preoperative smoking habit on rotator cuff tear: cigarette smoking influences rotator cuff tear sizes. J Shoulder Elbow Surg. 2012;21(1):56–60. [DOI] [PubMed] [Google Scholar]
- 6. Cavanaugh A, Rauh M, Thompson C, et al. Racial and ethnic disparities in utilization of total knee arthroplasty among older women. Osteoarthritis Cartilage. 2019;27(12):1746–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chaudhary MA, Sharma M, Scully RE, et al. Universal insurance and an equal access healthcare system eliminate disparities for Black patients after traumatic injury. Surgery. 2018;163(4):651–656. [DOI] [PubMed] [Google Scholar]
- 8. Csintalan RP, Inacio MC, Funahashi TT. Incidence rate of anterior cruciate ligament reconstructions. Perm J. 2008;12(3):17–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cutler DM, Lleras-Muney A. Understanding differences in health behaviors by education. J Health Econ. 2010;29(1):1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dunn WR, Wolf BR, Harrell FE, Jr, Reinke EK, Huston LJ, Spindler KP. Baseline predictors of health-related quality of life after anterior cruciate ligament reconstruction: a longitudinal analysis of a multicenter cohort at two and six years. J Bone Joint Surg Am. 2015;97(7):551–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Everhart JS, Best TM, Flanigan DC. Psychological predictors of anterior cruciate ligament reconstruction outcomes: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2015;23(3):752–762. [DOI] [PubMed] [Google Scholar]
- 12. Flanigan DC, Everhart JS, Glassman AH. Psychological factors affecting rehabilitation and outcomes following elective orthopaedic surgery. J Am Acad Orthop Surg. 2015;23(9):563–570. [DOI] [PubMed] [Google Scholar]
- 13. Gahagan JV, Hanna MH, Whealon MD, et al. Racial disparities in access and outcomes of cholecystectomy in the United States. Am Surg. 2016;82(10):921–925. [PubMed] [Google Scholar]
- 14. Goodman SM, Parks ML, McHugh K, et al. Disparities in outcomes for African Americans and whites undergoing total knee arthroplasty: a systematic literature review. J Rheumatol. 2016;43(4):765–770. [DOI] [PubMed] [Google Scholar]
- 15. Harris KK, Dawson J, Jones LD, Beard DJ, Price AJ. Extending the use of PROMs in the NHS—using the Oxford Knee Score in patients undergoing non-operative management for knee osteoarthritis: a validation study. BMJ Open. 2013;3(8):e003365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jain S, Schwarcz S, Katz M, Gulati R, McFarland W. Elevated risk of death for African Americans with AIDS, San Francisco, 1996-2002. J Health Care Poor Underserved. 2006;17(3):493–503. [DOI] [PubMed] [Google Scholar]
- 17. Karhade AV, Bono CM, Schwab JH, Tobert DG. Minimum clinically important difference: a metric that matters in the age of patient-reported outcomes. JBJS. 2021;103(24):2331–2337. [DOI] [PubMed] [Google Scholar]
- 18. Karim A, Pandit H, Murray J, Wandless F, Thomas NP. Smoking and reconstruction of the anterior cruciate ligament. J Bone Joint Surg Br. 2006;88(8):1027–1031. [DOI] [PubMed] [Google Scholar]
- 19. Kowalchuk DA, Harner CD, Fu FH, Irrgang JJ. Prediction of patient-reported outcome after single-bundle anterior cruciate ligament reconstruction. Arthroscopy. 2009;25(5):457–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lad SP, Bagley JH, Kenney KT, et al. Racial disparities in outcomes of spinal surgery for lumbar stenosis. Spine. 2013;38(11):927–935. [DOI] [PubMed] [Google Scholar]
- 21. Lad SP, Huang KT, Bagley JH, et al. Disparities in the outcomes of lumbar spinal stenosis surgery based on insurance status. Spine. 2013;38(13):1119–1127. [DOI] [PubMed] [Google Scholar]
- 22. Leathers MP, Merz A, Wong J, Scott T, Wang JC, Hame SL. Trends and demographics in anterior cruciate ligament reconstruction in the United States. J Knee Surg. 2015;28(5):390–394. [DOI] [PubMed] [Google Scholar]
- 23. Lucas FL, Stukel TA, Morris AM, Siewers AE, Birkmeyer JD. Race and surgical mortality in the United States. Ann Surg. 2006;243(2):281–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lyman S, Koulouvaris P, Sherman S, Do H, Mandl LA, Marx RG. Epidemiology of anterior cruciate ligament reconstruction: trends, readmissions, and subsequent knee surgery. J Bone Joint Surg Am. 2009;91(10):2321–2328. [DOI] [PubMed] [Google Scholar]
- 25. Martin CT, Callaghan JJ, Liu SS, Gao Y, Johnston RC. Disparity in preoperative patient factors between insurance types in total joint arthroplasty. Orthopedics. 2012;35(12):e1798–e1803. [DOI] [PubMed] [Google Scholar]
- 26. Mehta RH, Shahian DM, Sheng S, et al. Association of hospital and physician characteristics and care processes with racial disparities in procedural outcomes among contemporary patients undergoing coronary artery bypass grafting surgery. Circulation. 2016;133(2):124–130. [DOI] [PubMed] [Google Scholar]
- 27. Morris AM, Rhoads KF, Stain SC, Birkmeyer JD. Understanding racial disparities in cancer treatment and outcomes. J Am Coll Surg. 2010;211(1):105–113. [DOI] [PubMed] [Google Scholar]
- 28. Nelson CL. Disparities in orthopaedic surgical intervention. J Am Acad Orthop Surg. 2007;15(suppl 1):S13–S17. [DOI] [PubMed] [Google Scholar]
- 29. OME Cleveland Clinic. Implementing a scientifically valid, cost-effective, and scalable data collection system at point of care: the Cleveland Clinic OME cohort. JBJS. 2019;101(5):458–464. [DOI] [PubMed] [Google Scholar]
- 30. Perruccio AV, Stefan Lohmander L, Canizares M, et al. The development of a short measure of physical function for knee OA KOOS-Physical Function Shortform (KOOS-PS)—an OARSI/OMERACT initiative. Osteoarthritis Cartilage. 2008;16(5):542–550. [DOI] [PubMed] [Google Scholar]
- 31. Phyomaung PP, Dubowitz J, Cicuttini FM, et al. Are depression, anxiety and poor mental health risk factors for knee pain? A systematic review. BMC Musculoskelet Disord. 2014;15:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pierce RO, Jr. Ethnic and racial disparities in diagnosis, treatment, and follow-up care. J Am Acad Orthop Surg. 2007;15(suppl 1):S8–S12. [DOI] [PubMed] [Google Scholar]
- 33. Pierce TP, Elmallah RK, Lavernia CJ, et al. Racial disparities in lower extremity arthroplasty outcomes and use. Orthopedics. 2015;38(12):e1139–e1146. [DOI] [PubMed] [Google Scholar]
- 34. Ravi P, Sood A, Schmid M, et al. Racial/ethnic disparities in perioperative outcomes of major procedures. Ann Surg. 2015;262(6):955–964. [DOI] [PubMed] [Google Scholar]
- 35. Roos EM, Lohmander LS. The Knee injury and Osteoarthritis Outcome Score (KOOS): from joint injury to osteoarthritis. Health Qual Life Outcomes. 2003;1(1):64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD. Knee Injury and Osteoarthritis Outcome Score (KOOS)—development of a self-administered outcome measure. J Orthop Sports Phys Ther. 1998;28(2):88–96. [DOI] [PubMed] [Google Scholar]
- 37. Salim A, Ottochian M, DuBose J, et al. Does insurance status matter at a public, level I trauma center? J Trauma Acute Care Surg. 2010;68(1):211–216. [DOI] [PubMed] [Google Scholar]
- 38. Sanders TL, Maradit Kremers H, Bryan AJ, et al. Incidence of anterior cruciate ligament tears and reconstruction: a 21-year population-based study. Am J Sports Med. 2016;44(6):1502–1507. [DOI] [PubMed] [Google Scholar]
- 39. Schoenfeld AJ, Belmont PJ, Jr, See AA, Bader JO, Bono CM. Patient demographics, insurance status, race, and ethnicity as predictors of morbidity and mortality after spine trauma: a study using the National Trauma Data Bank. Spine J. 2013;13(12):1766–1773.e1761. [DOI] [PubMed] [Google Scholar]
- 40. Schoenfeld AJ, Tipirneni R, Nelson JH, Carpenter JE, Iwashyna TJ. The influence of race and ethnicity on complications and mortality after orthopedic surgery: a systematic review of the literature. Med Care. 2014;52(9):842–851. [DOI] [PubMed] [Google Scholar]
- 41. Selim AJ, Rogers W, Fleishman JA, et al. Updated U.S. population standard for the Veterans RAND 12-item Health Survey (VR-12). Qual Life Res. 2009;18(1):43–52. [DOI] [PubMed] [Google Scholar]
- 42. Singh JA, Lu X, Rosenthal GE, Ibrahim S, Cram P. Racial disparities in knee and hip total joint arthroplasty: an 18-year analysis of national Medicare data. Ann Rheum Dis. 2014;73(12):2107–2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Singh JA, Ramachandran R. Are there racial disparities in utilization and outcomes after total elbow arthroplasty? Rheumatol Int. 2015;35(9):1479–1487. [DOI] [PubMed] [Google Scholar]
- 44. Singh JA, Ramachandran R. Persisting racial disparities in total shoulder arthroplasty utilization and outcomes. J Racial Ethn Health Disparities. 2016;3(2):259–266. [DOI] [PubMed] [Google Scholar]
- 45. Spindler KP Huston LJ Wright RW; MOON Group Dunn WR, et al. The prognosis and predictors of sports function and activity at minimum 6 years after anterior cruciate ligament reconstruction: a population cohort study. Am J Sports Med. 2011;39(2):348–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Spindler KP, Warren TA, Callison JC, Jr, Secic M, Fleisch SB, Wright RW. Clinical outcome at a minimum of five years after reconstruction of the anterior cruciate ligament. J Bone Joint Surg Am. 2005;87(8):1673–1679. [DOI] [PubMed] [Google Scholar]
- 47. Taber DJ, Egede LE, Baliga PK. Outcome disparities between African Americans and Caucasians in contemporary kidney transplant recipients. Am J Surg. 2017;213(4):666–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yu S, Mahure SA, Branch N, Mollon B, Zuckerman JD. Impact of race and gender on utilization rate of total shoulder arthroplasty. Orthopedics. 2016;39(3):e538–e544. [DOI] [PubMed] [Google Scholar]
- 49. Zogg CK, Scott JW, Jiang W, Wolf LL, Haider AH. Differential access to care: the role of age, insurance, and income on race/ethnicity-related disparities in adult perforated appendix admission rates. Surgery. 2016;160(5):1145–1154. [DOI] [PubMed] [Google Scholar]