Abstract
Viral infectious diseases have various neurological manifestations, whether they are epidemic or pandemic in nature. Nonspecific encephalopathy is the most common central nervous system (CNS) manifestation. The spectrum of nervous evidence varies for viral pathogens. Some infectious viruses, such as the Ebola virus, exhibit direct neurotropism. Others, such as the Rift Valley fever virus, have the potential for neurotropism. Direct neurotropism is unknown in monkeypox virus, SARS-CoV-2, MERS-CoV, and even smallpox. As seen in the COVID-19, there may be evidence of para-infectious neurological syndrome. There have only been a few reports of neurological diseases caused by monkeypox infection. Future efforts to prevent the spread of infectious disease surges can reduce mortality complications, the therapeutic burden on the health-care system, and prevent further spread. This study describes the clinical and neurological complications of monkeypox infection, particularly encephalitis, as well as the laboratory diagnosis of these cases.
Keywords: Nervous system, Monkeypox virus, Neurology
1. Background
The name “monkeypox” appears on the World Health Organization (WHO) list of infectious diseases caused by viruses that have the potential to be endemic or pandemic, alongside Crimean-Congo hemorrhagic fever, Ebola virus disease, Hendra virus infection, influenza, Lassa fever, Marburg virus disease, MERS-CoV, SARS-CoV, Nipah virus infection, smallpox, yellow fever, Zika virus disease, SARS-CoV-2. These pathogens are becoming more common worldwide and typically cause neurological symptoms. Monkeypox virus is a re-emerging global health threat that has now posed unique challenges and can be expanded through borders. These consequences led to the WHO declaring hMPXV as the highest level of alert and a public health emergency of international concern on July 23, 2022 [1,2]. The COVID-19 pandemic demonstrated that pathogens with the potential for widespread spread could affect the nervous system [3]. Central neurologic states (meningitis, encephalitis, intraparenchymal hemorrhage, seizures), peripheral neurologic states (sensory neuropathy, sensorineural hearing loss, ophthalmoplegia), post-infectious states, and congenital states are all included in neurological observation (fetal microcephaly). Some diseases, including the monkeypox virus (MPXV) infection, have not been well defined neurologically, but they all have at least sporadic case reports of neurological features. Monkeypox can be considered as an emerging or re-emerging disease. Here, we highlight information about the cause, epidemiology, clinical neurological manifestations, and treatment of monkeypox infection.
2. Review methods
The criteria for including and excluding the study were searching in databases, including the NCBI database, Web of Science, CrossRef, Scopus, MEDLINE, PubMed, and Google Scholar. The search procedures were performed via essential keywords on neurological manifestations of the monkeypox virus and other orthopoxviruses.
3. Monkeypox epidemic disease
The exotic pet trade is one surprising example of emerging viral infections and the spread of associated diseases. The risk of strange imported animals introducing emerging pathogens increased dramatically in 2003 when a human MPXV (hMPXV) outbreak occurred in the United States [4]. The zoonotic monkeypox virus causes monkeypox, a rare viral infection. hMPXV is similar to smallpox but has milder symptoms and a lower mortality rate. There is no clear reservoir for it, but it appears to be transmitted by monkeys and terrestrial rodents such as mice and squirrels. Human-to-human transmission is possible (Fig. 1 ) [5]. The mortality rate from monkeypox infection varies depending on the virus clade. There is one death for every 100 infected people in the West African clade and one death for every ten infected people in the Central African clade. We cannot say with certainty whether the monkeypox infections observed in May 2022 have the potential to spread rapidly. On the other hand, while the virus is not highly contagious or infectious, it can cause severe complications in some people. These complications worsen in people with an immunodeficiency immune system or undergoing chemotherapy, increasing the hospitalization rate. These include chest infections, neurological complications such as encephalitis, sepsis, and even eye infections and blindness [6]. In the most recent studies, there are rare cases of monkeypox neurological complications. The patients were young girls with encephalitis (brain inflammation caused by viral infections) who needed to be ventilated. The first case involved a three-year-old girl who died on the second day of her hospitalization. The second girl, a 6-year-old girl, made it through 14 days in the intensive care unit (ICU) [7,8]. The second patient had orthopoxviral-reactive IgM in her CSF, but the first had no diagnosis. Recent clinical manifestations of monkeypox in different human hosts may be the same due to exposure to the same virus strain, all of which have a common source. Except for the varicella vaccine, her childhood vaccinations were up to date. CSF culture and PCR results on skin lesions were both positive. The patient eventually recovered over time.
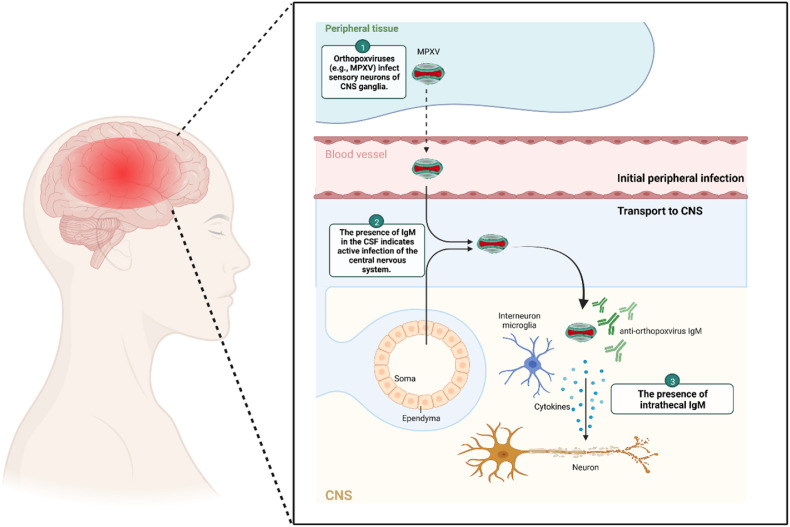
Fig. 1.
The entry routes of orthopoxviruses into the CNS. Microglia macrophages provide immune monitoring in CNS. Orthopoxviruses find their way into the CNS (either through direct nerve infection in tissues or by infecting cells in the circulatory system). They eventually transmit the infection through the blood-brain barrier to the CNS.
4. Neurological complications
The following neurological symptoms have been reported for monkeypox-associated encephalitis: fever, pharyngitis, anorexia, weakness, headache, adenopathy, and a vesiculopapular rash. Within 24 h, the rash has spread to the face, trunk, extremities, palms, and soles. The cortical MRI data show diffuse edema, meningeal amplification, and signal abnormalities in the thalamus and partial cortex, as well as a very slow electroencephalogram (EEG). Cerebrospinal fluid (CSF) has predominantly polymorphonuclear pleocytosis with normal average glucose and protein levels; however, pleocytosis may decrease and primarily include lymphocytes within 5–6 days [7]. The vaccinia virus is the most studied orthopoxvirus prototype species, and it is used as a live attenuated virus in the smallpox vaccine. Despite its success, several vaccine strain-dependent complications, such as eczema vaccinatum, progressive vaccinia, and neurological complications, have been reported [9]. The risk of encephalitis following Smallpox vaccination is well known. Possible diagnosis of encephalitis usually occurs based on the onset of an acute illness, 7–14 days after vaccination, with fever, headache, vomiting, mental condition change, meningeal irritation, paralysis, or dizziness. Numerous reports of encephalitis following smallpox vaccination show that the incidence varies not only in different locations but also in the same place in different years. However, severe neurological side effects, such as post-vaccination encephalitis and Gillen Barre syndrome, have been occurred by the expected range [10].
5. Other findings so far
Prior to April 2022, the hMPXV infection was native to Africa and rarely spread outside. Although the mortality rate is significantly different in both Central Africa and Congo clades, the deaths of young children and people with HIV were the most likely. The common hMPXV transmission was either imported, household, or nosocomial. But the alarm of the release of the hMPXV continued on May 6, 2022, with a report from a British national after returning from Nigeria, and to date (July 25, 2022), according to WHO reports, more than 16000 affected by most countries have been reported for the highest level of alert considered [[11], [12], [13]]. As is evident, the clinical state is marked by fever, skin rash (papules, vesicles, pustules, ulcerating lesions, and scabs), and lymphadenopathy (%56). Severe complications include pneumonitis, encephalitis, sight-threatening keratitis, and secondary bacterial infections. In a more recent study [14], viremia, or extended upper respiratory tract viral DNA shedding following skin lesion resolution, was found in seven British patients with hMPXV, challenging current infection prevention and control recommendations (with the expected liver enzyme disorder during treatment). Brincedofovir and tacoberimate are two oral medications that have been licensed for the treatment of monkeypox in animals. Patients receiving the second therapy did not have any unfavorable side effects, and even viral shedding and the duration of the disease following treatment were reduced compared to those receiving the first (10 days in the hospital). The former, however, efficacy less well. Antiviral research is crucial for the hMPXV. According to the reports, the UK Health Security Agency has classed hMPXV as an infectious disease with a high consequence. It is noteworthy that none of the patients experienced the severe and well-known complications of hMPXV, although in one case, a mild recurrence was discharged from the hospital. In another study, 98% of the 528 hMPXV infections recorded in the 16 countries were gay or bisexual, and 41% of those infected also had HIV. The possibility of sexual transmission was verified in 95% of cases, making it the most likely transmission route during the recent hMPXV incidence. Yet, it's important to remember that hMPXV is not just a homosexual disease [15,16]. The shift in human behavior and lifestyle, as well as the biological characteristics of the virus, are responsible for the current global predominance of monkeypox virus. Although the most frequent causes of hospitalization were nearly the same as before, a broad range of disease consequences, including new and rare complications like myocarditis, should be noted and followed up. Since viral infections can reemerge at any time, we must be mindful of the risks they pose in the future.
The discovery of a common zoonotic reservoir can help because of the main cause of the current outbreak. It should help to explain how epidemiological data, zoonotic nature, and analysis of earlier prevalence reports are related [17]. Most of the current cases have occurred in the male population (gay or bisexual) who do not have a history of traveling to the endemic (with an average age of 20–49 years). In addition, this virus has been isolated only from other species, such as giant poached rats, rope squirrels, and monkeys [18].
6. Regulatory profile of monkeypox virus
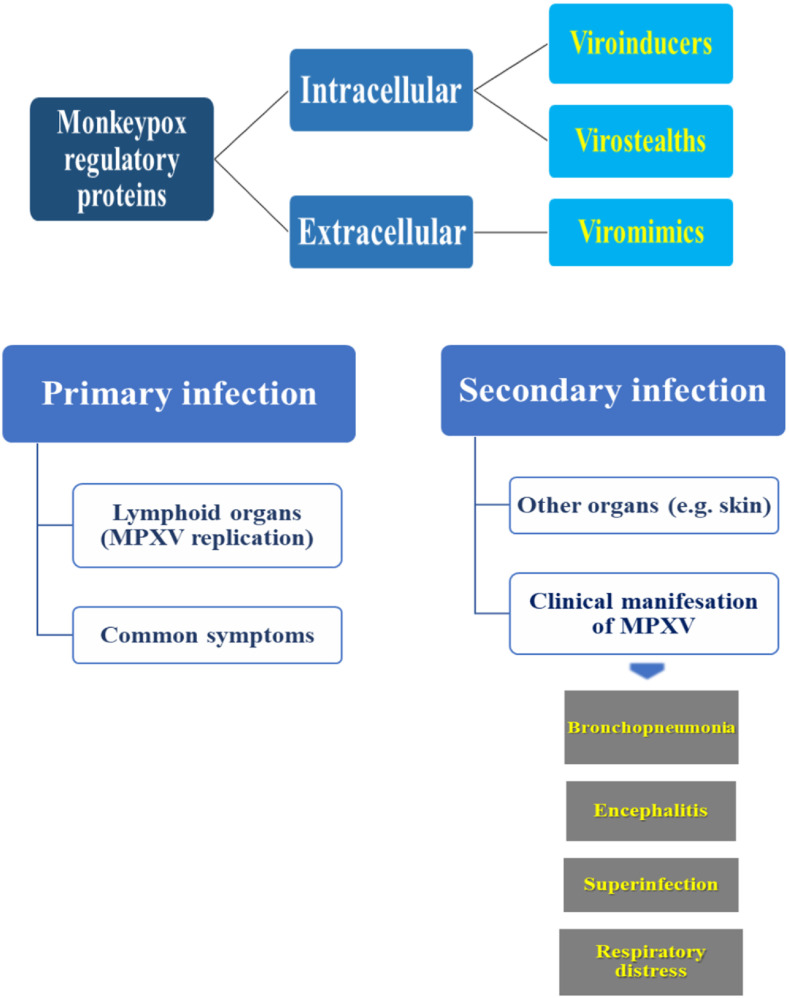
The intracellular proteins of the monkeypox virus either cause interference in the cellular response to infection through cellular pathways such as apoptosis (viroinducers) or through intracellular ways the virus escapes from the host's immune system and major histocompatibility complex class 1 (MHC 1) (virostealths). Extracellular proteins also modulate the host's immune system in favor of the MPXV. They mimic the role of glycoproteins and competitively bind to host cytokines and interfere with their function. This group not only modulates the immune response against the virus but also causes the virus to replication and spread better. In primary infection, the viral load distributes to local lymph nodes. In secondary infection, the viral load reaches distant organs via circulation. The incubation period usually lasts 7–14 days. In cases before the 2022 outbreak, the rash appeared one to three days after the onset of fever and lymphadenopathy. Still, the new cases for some patients show that some people may not know any symptoms until the rash appears [19,20]. The common symptoms and complications develop 1–2 weeks after people have been infected with MPXV. Common symptoms include fever, headache, back pain, myalgia, fatigue, inflammation/lymphadenopathy, and skin rash (Fig. 2 ). Without these regulatory proteins, MPXV cannot escape the immune system and develop complications in the hosts.
Fig. 2.
Suggested regulatory proteins and pathogenesis of Monkeypox virus.
7. The neurologic manifestation in monkeypox infection: severe, mild or rare?
In recent years, the Democratic Republic of the Congo has recorded over 500 suspected cases of human disease prevalence. Monkeypox virus is a double-stranded DNA virus in the Orthopoxvirus genus. It differs genetically from other orthopoxviruses, such as the variola virus (which causes smallpox) and the vaccinia virus (the virus used as the smallpox vaccine) [8,21]. Secondary transmission events have increased over time as the number of people who have never been immunized against smallpox has increased. We are currently seeing cases of monkeypox infection. Adenopathy is a typical feature of the monkeypox virus from smallpox. MPXV lesions should be distinguished from lesions caused by other viral infections that cause encephalitis (such as a varicella-zoster virus). VZV lesions are typically small and superficial, primarily on the trunk, and develop in stages [22]. Encephalitis has rarely been associated with the complication of MPXV (as described above). Vaccinia virus is an Orthopoxvirus associated with postvaccinial encephalomyelitis (PVE). PVE cases are observed when administered as a vaccine against smallpox [23]. PVE patients have different clinical and diagnostic features, including acute disseminated encephalomyelitis post-immunization or direct viral infection of the central nervous system [24]. In the clinical manifestations of smallpox, encephalopathy is expected. Severe headaches, hallucinations, and delirium are also common complaints. Smallpox can also cause serious ocular complications. In patients infected with smallpox, mortality from encephalitis can be as high as 25% after vaccination. Other neurological complications linked to the vaccine include headache, seizures, cranial nerve palsy, Guillain-Barre syndrome, hemiplegia, and coma [25]. Skin biopsy and encephalopathy confirmed the diagnosis of monkeypox-associated encephalitis in the described patient. Because IgM does not generally cross the blood-brain barrier, the presence of IgM in CSF indicates active central nervous system infection with intrathecal antibody production (laboratory diagnostic standard). The absence of demyelination, cytotoxic changes caused by diffuse and focal edema, and intrathecal antibodies (IgM) all point to monkeypox as a possible cause of acute encephalitis [26].
8. Differential diagnostic tests
In the differential diagnosis of monkeypox infection, doctors should consider encephalitis cases with inflammation in the presence of atypical vesicopustular rash. In patients with a skin rash, the common causes of viral encephalitis-associated inflammation, particularly varicella infection, should be considered in the differential diagnosis of encephalitis. In addition to the above, in the case of monkeypox infection, the history of travel to Africa or exposure to exotic pets should be investigated. A range of new diagnostic methods can be used for orthopoxvirus infections, including PCR, serological tests, and Immunohistochemistry (IHC). The anti-orthopoxvirus IgM reaction in CSF may be valuable in diagnosing orthopoxvirus-induced encephalitis. This process accelerates via seroconversion from the anti-orthopoxvirus IgM response to the anti-orthopoxvirus IgG response [7,27].
9. Treatment
There are several treatment options for monkeypox infection. Cidofovir, an antiviral drug, is effective against poxviruses such as monkeypox and vaccina viruses. This drug has been described as a second-line treatment for smallpox vaccine side effects. Due to serological cross-protection between orthopoxviruses, vaccinia immune globulin (VIG) can effectively treat monkeypox infection. Along with other treatment options, supportive care is recommended. Tecovirimat is another antiviral drug, that has been shown to be effective against orthopoxviruses in vitro and is safe to use in humans. It has never been given to people who have been infected with MPXV before. Monkeypox and smallpox can both be prevented with the JYNNEOS vaccine. ACAM2000 is a more recent vaccine that can be given to people over the age of 18 who are at high risk of contracting smallpox. Because the viruses that cause smallpox and monkeypox are so similar, the smallpox vaccine can also protect people from getting monkeypox [28,29].
Despite the above, supportive therapy is the mainstay of management of monkeypox virus infection. Although the symptomatic management and prevention of complications are proper, there is no apparent cure for monkeypox. For example, in fever, respiratory distress/bronchopneumonia, and skin superinfection should use antipyretic medications, external cooling, or oral/intravenous antibiotics, respectively. Inflammation/lymphadenopathy should be treated with oral/intravenous anti-inflammatory.
10. Conclusion and prospective
Our understanding of emerging infectious diseases is currently limited. Because of what humans learned during the COVID-19 pandemic, we can confidently predict that the list of emerging pathogens that cause human disease will continue to grow. Changes in the ecology and climate, as well as the distribution of disease-transmitting vectors and their hosts, increased the risk of diseases-transmitting vectors for humans. The predictable emergence of opportunistic infections is the price of advances in infectious disease science. However, we can develop newer disease prevention and treatment strategies as we learn more about the host immune response and pathogen replication.
Funding
No funding.
Consent statement
Not applicable.
Data availability statement
Not applicable.
CRediT authorship contribution statement
Maryam Shafaati: Writing – original draft, Preparation, All authors have read and agreed to the published version of the manuscript. Milad Zandi: Conceptualization, Writing – review & editing, Editing the manuscript for revision, Supervision, All authors have read and agreed to the published version of the manuscript.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Acknowledgments
Not applicable.
References
- 1.Grover R., Rigby WHO declares global health emergency over monkeypox outbreak. 2022. https://www.reuters.com/business/healthcare-pharmaceuticals/monkeypox-outbreak-constitutes-global-health-emergency-who-2022-07-23/ [Available from:
- 2.Al-Tawfiq J.A., Barry M., Memish Z.A. International outbreaks of Monkeypox virus infection with no established travel: a public health concern with significant knowledge gap. Trav Med Infect Dis. 2022 doi: 10.1016/j.tmaid.2022.102364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.(Who WHO. emergencies/diseases. 2022. https://www.who.int/emergencies/diseases [Available from:
- 4.Reed K.D., Melski J.W., Graham M.B., Regnery R.L., Sotir M.J., Wegner M.V., et al. The detection of monkeypox in humans in the Western Hemisphere. N Engl J Med. 2004;350(4):342–350. doi: 10.1056/NEJMoa032299. [DOI] [PubMed] [Google Scholar]
- 5.Heymann D.L., Szczeniowski M., Esteves K. Re-emergence of monkeypox in Africa: a review of the past six years. Br Med Bull. 1998;54(3):693–702. doi: 10.1093/oxfordjournals.bmb.a011720. [DOI] [PubMed] [Google Scholar]
- 6.Vakharia D.K. Monkeypox virus causes, symptoms, and treatment. 2022. https://patient.info/infections/monkeypox-virus [Available from:
- 7.Sejvar J.J., Chowdary Y., Schomogyi M., Stevens J., Patel J., Karem K., et al. Human monkeypox infection: a family cluster in the midwestern United States. J Infect Dis. 2004;190(10):1833–1840. doi: 10.1086/425039. [DOI] [PubMed] [Google Scholar]
- 8.Ježek Z., Szczeniowski M., Paluku K., Mutombo M. Human monkeypox: clinical features of 282 patients. J Infect Dis. 1987;156(2):293–298. doi: 10.1093/infdis/156.2.293. [DOI] [PubMed] [Google Scholar]
- 9.Smith G.L. Springer; Poxviruses: 2007. Genus Orthopoxvirus: vaccinia virus; pp. 1–45. [Google Scholar]
- 10.Sejvar J.J., Labutta R.J., Chapman L.E., Grabenstein J.D., Iskander J., Lane J.M. Neurologic adverse events associated with smallpox vaccination in the United States, 2002-2004. JAMA. 2005;294(21):2744–2750. doi: 10.1001/jama.294.21.2744. [DOI] [PubMed] [Google Scholar]
- 11.Kumar N., Acharya A., Gendelman H.E., Byrareddy S.N. The 2022 outbreak and the pathobiology of the monkeypox virus. J Autoimmun. 2022 doi: 10.1016/j.jaut.2022.102855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization Monkeypox. 2019 https://www.who.int/emergencies/situation-reports [updated July 25, 2022]. Available from: [Google Scholar]
- 13.Mileto D., Riva A., Cutrera M., Moschese D., Mancon A., Meroni L., et al. New challenges in human monkeypox outside Africa: a review and case report from Italy. Trav Med Infect Dis. 2022 doi: 10.1016/j.tmaid.2022.102386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adler H., Gould S., Hine P., Snell L.B., Wong W., Houlihan C.F., et al. The Lancet Infectious Diseases; 2022. Clinical features and management of human monkeypox: a retrospective observational study in the UK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thornhill J.P., Barkati S., Walmsley S., Rockstroh J., Antinori A., Harrison L.B., et al. Monkeypox virus infection in humans across 16 countries—april–june 2022. N Engl J Med. 2022 doi: 10.1056/NEJMoa2207323. [DOI] [PubMed] [Google Scholar]
- 16.Walter K., Malani P.N. What is monkeypox? JAMA. 2022 doi: 10.1001/jama.2022.10259. [DOI] [PubMed] [Google Scholar]
- 17.Bhattacharya M., Dhama K., Chakraborty C. Recently spreading human monkeypox virus infection and its transmission during COVID-19 pandemic period: a travelers' prospective. Trav Med Infect Dis. 2022 doi: 10.1016/j.tmaid.2022.102398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farahat R.A., Ali I., Al-Ahdal T., Benmelouka A.Y., Albakri K., El-Sakka A.A., et al. 2022. Monkeypox and human transmission: are we on the verge of another pandemic? Travel medicine and infectious disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okyay R.A., Bayrak E., Kaya E., Şahin A.R., Koçyiğit B.F., Taşdoğan A.M., et al. Another epidemic in the shadow of covid 19 pandemic: a review of monkeypox. Proteins. 2022;7:10. [Google Scholar]
- 20.Petersen E., Kantele A., Koopmans M., Asogun D., Yinka-Ogunleye A., Ihekweazu C., et al. Human monkeypox: epidemiologic and clinical characteristics, diagnosis, and prevention. Infectious Disease Clinics. 2019;33(4):1027–1043. doi: 10.1016/j.idc.2019.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luna N., Ramírez A.L., Muñoz M., Ballesteros N., Patiño L.H., Castañeda S.A., et al. Phylogenomic analysis of the monkeypox virus (MPXV) 2022 outbreak: emergence of a novel viral lineage? Trav Med Infect Dis. 2022 doi: 10.1016/j.tmaid.2022.102402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arvin A.M., Gershon A.A. Cambridge University Press; 2000. Varicella-zoster virus: virology and clinical management. [Google Scholar]
- 23.Goldstein J.A., Neff J.M., Lane J.M., Koplan J.P. Smallpox vaccination reactions, prophylaxis, and therapy of complications. Pediatrics. 1975;55(3):342–347. [PubMed] [Google Scholar]
- 24.Tenembaum S., Chamoles N., Fejerman N. Acute disseminated encephalomyelitis: a long-term follow-up study of 84 pediatric patients. Neurology. 2002;59(8):1224–1231. doi: 10.1212/wnl.59.8.1224. [DOI] [PubMed] [Google Scholar]
- 25.McEntire C.R., Song K.-W., McInnis R.P., Rhee J.Y., Young M., Williams E., et al. Neurologic manifestations of the World Health Organization's list of pandemic and epidemic diseases. Front Neurol. 2021;12:161. doi: 10.3389/fneur.2021.634827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tyler K.L. Emerging viral infections of the central nervous system: part 2. Arch Neurol. 2009;66(9):1065–1074. doi: 10.1001/archneurol.2009.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tyler K.L. Emerging viral infections of the central nervous system: part 1. Arch Neurol. 2009;66(8):939–948. doi: 10.1001/archneurol.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Downie A., McCarthy K. The viruses of Variola, vaccinia, cowpox and ectromelia.—neutralization tests on the chorio-allantois with unabsorbed and absorbed immune sera. Br J Exp Pathol. 1950;31(6):789. [PMC free article] [PubMed] [Google Scholar]
- 29.De Clercq E. Cidofovir in the treatment of poxvirus infections. Antivir Res. 2002;55(1):1–13. doi: 10.1016/S0166-3542(02)00008-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.