Figure 1.
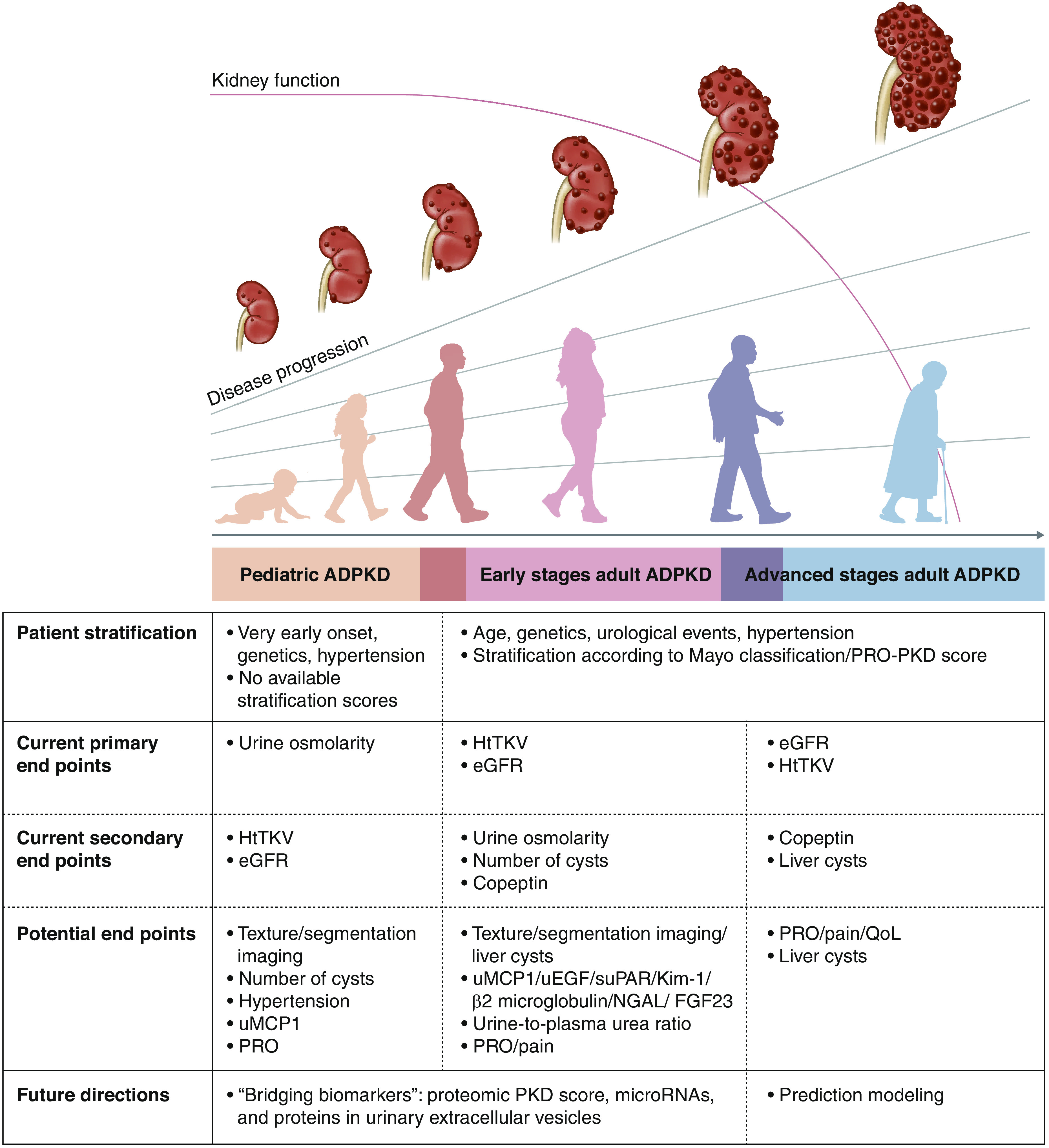
Published study end points for patients with autosomal dominant polycystic kidney disease (ADPKD) include height-adjusted total kidney volume (htTKV) and eGFR in adults and urinary osmolality in children. As GFR remains stable for years, other progression markers are needed for future clinical trials. Mayo classification (1A–1E) and/or the Predicting Renal Outcomes in Polycystic Kidney Disease (PRO-PKD) tool may be used for stratifying patients for selection in trials. Copeptin, kidney cyst number, and liver cysts are secondary end points used in previous studies, but all are not validated. Urinary monocyte chemoattractant protein-1 (uMCP1), kidney injury molecule-1 (Kim-1), β2-microglobulin, neutrophil gelatinase–associated lipocalin (NGAL), fibroblast growth factor 23 (FGF23), urinary epidermal growth factor (uEGF), soluble urokinase plasminogen activator receptor (suPAR), urine-plasma urea ratio, and patient-reported outcomes (PROs), such as pain, are potential biomarkers. Future directions in ADPKD including “bridging biomarkers,” such as microRNAs, proteomics, and proteins in urinary extracellular vesicles, are promising. Automatic segmentation using artificial intelligence/machine learning may further enhance the interpretation of kidney growth, and number and size of cysts. Combining all of these efforts will lead to the development of prediction modeling with a more precise disease progression stratification. PKD, polycystic kidney disease; QoL, quality of life.
