Significance Statement
In this study of people with probable CKD accessing health care, we observed profound sex differences in detection, recognition, and monitoring that persisted over time: women were less likely to receive a CKD diagnosis, to visit a nephrologist, to have their creatinine and albuminuria measured, or to receive guideline-recommended therapies. Differences in comorbidity did not explain these discrepancies and were similar among high-risk groups, among patients with evidence-based indications for medications, and among patients with confirmed CKD at retesting. Efforts to improve and ensure equitable health care between the sexes could have important implications for justice and could reduce the burden of CKD.
Keywords: chronic kidney disease, sex difference, epidemiology and outcomes
Visual Abstract
Abstract
Introduction
Reported sex differences in the etiology, population prevalence, progression rates, and health outcomes of people with CKD may be explained by differences in health care.
Methods
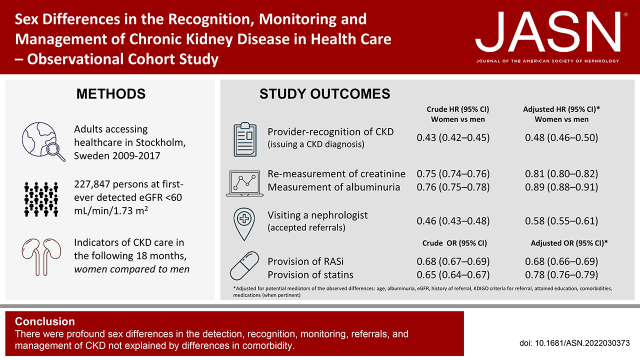
We evaluated sex as the variable of interest in a health care–based study of adults (n=227,847) with at least one outpatient eGFR<60 ml/min per 1.73 m2 measurement denoting probable CKD in Stockholm from 2009 to 2017. We calculated the odds ratios for diagnosis of CKD and provision of RASi and statins at inclusion, and hazard ratios for CKD diagnosis, visiting a nephrologist, or monitoring creatinine and albuminuria during follow-up.
Results
We identified 227,847 subjects, of whom 126,289 were women (55%). At inclusion, women had lower odds of having received a diagnostic code for CKD and were less likely to have received RASi and statins, despite having guideline-recommended indications. In time-to-event analyses, women were less likely to have received a CKD diagnosis (HR, 0.43; 95% CI, 0.42 to 0.45) and visited a nephrologist (HR, 0.46; 95% CI, 0.43 to 0.48) regardless of disease severity, presence of albuminuria, or criteria for referral. Women were also less likely to undergo monitoring of creatinine or albuminuria, including those with diabetes or hypertension. These differences remained after adjustment for comorbidities, albuminuria, and highest educational achievement, and among subjects with confirmed CKD at retesting. Although in absolute terms all nephrology-care indicators gradually improved over time, the observed sex gap persisted.
Conclusions
There were profound sex differences in the detection, recognition, monitoring, referrals, and management of CKD. The disparity was also observed in people at high risk and among those who had guideline-recommended indications.
CKD is prevalent, affecting 8%–16% of adults worldwide (11.8% of all women, 10.4% of all men), conferring large health care costs, and contributing to excess comorbidity and death.1–4 There are important sex differences in the etiology, prevalence, progression rates, and outcomes of people with CKD. Although most people with CKD are identified, followed, and managed in the primary care setting,5 evidence on differences by sex or gender derive mostly from the minority of patients referred to nephrology specialist units. These studies show that more women than men have CKD,6 but more men than women undergo KRT.7–10 It is controversial whether this is because men progress more rapidly to kidney failure,11–13 because women more often opt for conservative care,14 or whether social factors are involved.15 It is possible that differences in clinical care may contribute to poorer outcomes: women undergoing dialysis have greater hospitalization rates,16,17 excess deaths, worse relative survival, and more years of life lost than men.18 Conscious or unconscious bias may be operating and leading to differential management by sex or gender.
Guidelines emphasize referral to nephrologist care according to the principle of risk stratification,1,19,20 the presence of specific comorbidities and cutpoints in eGFR and albuminuria that are not sex based.1,19,20 Whether systematic differences exist in process indicators has not been systematically addressed, but secondary evidence suggests they may occur: in the United Kingdom, mean GFR in men who had been referred was higher than that in women,21 and women were less likely to receive a diagnostic code of CKD.22 A Canadian cross-sectional study of people with CKD in primary care23 reported less testing of urine albumin-creatinine ratio in women; and in the United States, awareness of having CKD24 or the likelihood of initiating renin-angiotensin inhibitors (RASi) on detection of incident albuminuria25 was lower among women. The objective of this study was to explore differences in the recognition, detection, monitoring, and management of CKD between men and women in the contemporary routine healthcare of Stockholm, Sweden.
Materials and Methods
Study Setting
We used data from the Stockholm Creatinine Measurements (SCREAM) project, a health care utilization cohort from the region of Stockholm, Sweden.26 A single health care provider in the Stockholm region provides universal and tax-funded health care to 20%–25% of the population of Sweden. Using unique personal identification numbers, SCREAM linked regional and national administrative databases that hold complete information on demographics, health care utilization, laboratory tests undertaken, dispensed drugs, diagnoses, and vital status, without loss to follow-up. The Regional Ethical Review Board in Stockholm and the Swedish National Board of Welfare approved the study. Because data were linked and deidentified by the Swedish government, informed consent was not deemed necessary.
Study Population
We extracted information on all serum or plasma creatinine measurements performed in outpatient care during 2009–2018 for any citizen of adult age (≥18 years), and calculated eGFR using the 2009 CKD Epidemiology Collaboration equation without correction for race.27 During this period, all laboratories used creatinine assays calibrated to isotope-dilution mass spectrometry and were subject to frequent quality audits.28 We identified all individuals who had at least one eGFR measurement <60 ml/min per 1.73 m2, denoting probable CKD category G3a or worse according to Kidney Disease: Improving Global Outcomes (KDIGO).1 The first time that a low eGFR was encountered in our system constituted the index date. At that point, we excluded people with a history of KRT and those with missing information on age or sex. To explore time trends, we repeated this process for each calendar year, creating a series of nine cohorts of individuals with a least one detected eGFR<60 ml/min per 1.73 m2 during each year (Supplemental Figure 1). A given individual could be present in several of these cohorts if they underwent creatinine testing and fulfilled inclusion criteria.
Exposure and Covariates
The variable of interest was registered sex, identified in each citizen’s unique identity number.29 Study covariates included age, comorbidities, ongoing medications (all detailed in Supplemental Table 1), eGFR, albuminuria, and indicators of nephrology care. We identified comorbidities through clinical diagnosis codes, and ascertained medications through drug dispensations at registered pharmacies. The highest-educational attainment for each participant was obtained by linkage with the government-run longitudinal integrated database for health insurance and labor market studies registry,30 and categorized as compulsory school, secondary school, and university education. We classified the severity of CKD using KDIGO G categories on the basis of index eGFR.1 We evaluated the presence and severity of albuminuria through extraction of all tests of dipstick albuminuria/proteinuria, urine albumin-creatinine ratio, and albuminuria excretion rates, and categorization into KDIGO categories A1–A3.1 Finally, we evaluated whether individuals satisfied KDIGO criteria1 or regional criteria set by Region Stockholm for referral to nephrology care. KDIGO criteria for referral included eGFR <30 ml/min per 1.73 m2, presence of A3 albuminuria, or CKD with refractory hypertension. Regional criteria for referral were eGFR<60 ml/min per 1.73 m2 and age <50 years; albuminuria A3 and age 50–80 years; albumin-creatinine ratio >100 mg/mmol and age >80 years; or eGFR<30 ml/min per 1.73 m2 and age >80 years.
Outcomes
We evaluated key steps in the process of CKD care, including provider recognition (i.e., issuing a diagnosis), monitoring (i.e., remeasurement of creatinine and albuminuria), referral to nephrologist care, and management (provision of guideline-recommended medications). Some of these are described cross-sectionally at the index date, as a proportion, on the basis of lookback periods; others as a time-to-event during follow-up. We defined having ever received a diagnosis of CKD or having had a nephrologist visit by evaluating administrative records from each patient since 1997; and reported time-to-event data during 18-month follow-up on those who did not meet this criterion. We defined having creatinine remeasured or albuminuria measured in time-to-event data for the next 18 months. Finally, we defined guideline-recommended use of RASi and statins on the basis of pharmacy dispensations claimed at index date and the 6 months before or after. Individuals were censored at death, emigration from the Stockholm region, or end of follow-up (December 2019).
Statistical Analysis
Descriptive statistics for categorical variables were presented with counts (%), and continuous variables by mean and SD, or median and interquartile range. We calculated absolute proportions and used univariable logistic regression, presenting odds ratios (OR) with 95% confidence intervals (95% CI), to explore cross-sectional sex differences at the index date. For time-to-event data, we used the Aalen–Johansen approach to estimate cumulative incidence functions (censoring at migration, death, and end of follow-up) and univariable Cox proportional hazards models, presenting hazard ratios (HR) with 95% CI.
Because the relationship between sex and outcomes cannot be confounded by other variables—it is not possible for other variables to causally influence sex—we present crude risks. Next, we used multivariable adjustment to explore if differences in nephrology care were explained by underlying risk factors or comorbidity, which we interpret as potential mediators of the associations under study. Multivariable adjustment included (when applicable): age, albuminuria, eGFR, prior referral, presence of KDIGO criteria for referral, highest educational attainment, hypertension, diabetes mellitus, myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic obstructive pulmonary disease, and cancer.
Analyses were performed overall and in subgroups of age, presence of specific comorbidities, CKD category, albuminuria, and people with at least one criterion for nephrology referral. In the analysis of medication use, we explored subgroups across specific indications stressed by guidelines.1 As supporting analyses, we evaluated CKD care indicators in situations where we hypothesized that sex differences would be less evident: (1) people with a history of nephrologist referral; (2) people with eGFR<60 ml/min per 1.73 m2 at the point of retesting (that is, those with confirmed CKD); and (3) people identified before or after the implementation of automatic eGFR reporting in our region, which occurred at the start of 2015.
As sensitivity analyses, we tested whether further adjustment for creatinine abrogated associations and whether study results differed by applying a different eGFR equation (the Lund–Malmö equation), which was used in the automatic eGFR reporting after 2015.31 Finally, in the evaluation of time trends, we compared nephrology care indicators using men in year 2009 as a reference. Analyses were performed using R project version 4.0.3 and Stata version 16 software (StataCorp).
Results
From 2009 to 2017, 227,847 residents had at least one outpatient creatinine measurement equivalent to an eGFR <60 ml/min per 1.73 m2 and fulfilled inclusion criteria (Supplemental Figure 1). Over half of the participants were women (55%) and median age was higher in women compared with men (77 versus 74 years, Supplemental Table 2). Mean eGFR was similar for both sexes (51 ml/min per 1.73 m2), and most people had CKD G3a (80%), followed by G3b (15%), G4 (4%), and G5ND (1%). Despite being older, women generally had a lower comorbidity burden; a higher proportion of men had diabetes (23% of men versus 17% of women) and cardiovascular diseases (e.g., myocardial infarction in 15% of men versus 8% of women, and peripheral arterial disease in 10% versus 6%), but the proportion with hypertension was similar (62% versus 63%, respectively). A high proportion of the individuals lacked albuminuria testing at baseline, but among those who had, women had lower albuminuria than men (Supplemental Table 2).
During each calendar year, there were 70,000 to 80,000 unique individuals with at least one eGFR measurement <60 ml/min per 1.732. The characteristics of patients in these calendar-year cohorts are shown in Supplemental Table 3 and resemble that of the overall cohort.
Diagnosis of CKD
At cohort inclusion, few people (5%) had ever received a CKD diagnosis code. This was more common among men (6.9% of men versus 3.4% of women). Women had lower odds of having received a CKD diagnosis compared with men (unadjusted OR, 0.47; 95% CI, 0.45 to 0.49, Supplemental Table 4), a difference that was observed across all subgroups evaluated. Compared with men, women had lower odds of having received a CKD diagnosis across severity categories G3a, G3b, and G4, but not among patients with G5ND. This persisted, although attenuated, by multivariate adjustment to OR 0.55 (95% CI, 0.53 to 0.58).
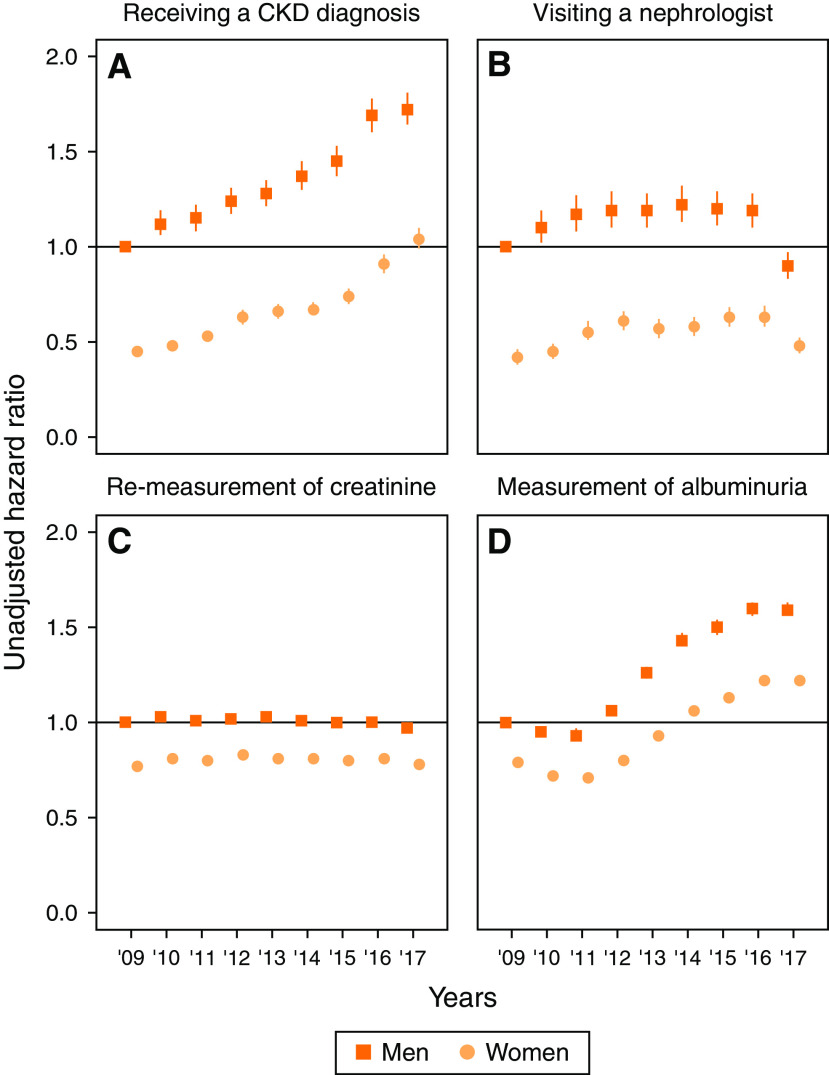
Within the next 18 months, fewer women than men received an incident diagnosis of CKD (7.5% of men versus 3.4% of women), with a HR of 0.43 and 95% CI, 0.42 to 0.45 (Table 1). This difference was observed across all subgroups, including G5ND, and persisted after multivariable adjustment (e.g., adjusted HR, 0.48; 95% CI, 0.46 to 0.50 in the overall cohort). In time-trend analyses, the proportion of diagnosed patients increased each calendar year, and the underdiagnosis of women persisted (Figure 1A, Supplemental Table 5).
Table 1.
Cumulative incidence and HR of receiving a CKD diagnosis in the next 18 months after baseline among people without a CKD diagnosis at inclusion, stratified by sex at first eGFR measurement <60 ml/min per 1.73 m2 in the Stockholm region from 2009 to 2017
| Category | 18-Month Cumulative Incidence Estimates (95% CI) of CKD Diagnosis | HR (95% CI) of Receiving an Incident CKD Diagnosis in the Next 18 Months | |||||
|---|---|---|---|---|---|---|---|
| Number of Individuals | Men | Women | Unadjusted HR Women versus Men |
P Value | Adjusted HRd Women versus Men |
P Value | |
| Overall | 216,519 | 7.5% (7.4 to 7.7) | 3.4% (3.3 to 3.5) | 0.43 (0.42 to 0.45) | <0.001 | 0.48 (0.46 to 0.50) | <0.001 |
| By age categories, yr | |||||||
| <65 | 43,503 | 8.9% (8.5 to 9.3) | 4.4% (4.1 to 4.6) | 0.47 (0.44 to 0.51) | <0.001 | 0.56 (0.52 to 0.60) | <0.001 |
| 65–75 | 61,416 | 7.0% (6.7 to 7.3) | 3.0% (2.8 to 3.1) | 0.41 (0.38 to 0.44) | <0.001 | 0.49 (0.45 to 0.53) | <0.001 |
| >75 | 111,600 | 7.2% (6.9 to 7.4) | 3.3% (3.2 to 3.4) | 0.43 (0.41 to 0.46) | <0.001 | 0.42 (0.40 to 0.45) | <0.001 |
| By presence of comorbidities | |||||||
| Hypertension | 133,012 | 8.2% (8.0 to 8.4) | 3.8% (3.7 to 4.0) | 0.45 (0.43 to 0.47) | <0.001 | 0.50 (0.48 to 0.53) | <0.001 |
| Diabetes | 40,441 | 10.9% (10.5 to 11.3) | 5.8% (5.5 to 6.1) | 0.51 (0.47 to 0.55) | <0.001 | 0.55 (0.51 to 0.60) | <0.001 |
| CVD | 76,720 | 8.7% (8.5 to 9.0) | 4.6% (4.3 to 4.8) | 0.50 (0.48 to 0.53) | <0.001 | 0.49 (0.47 to 0.53) | <0.001 |
| By CKD category | |||||||
| G3a | 177,212 | 5.3% (5.1 to 5.5) | 2.0% (1.9 to 2.1) | 0.36 (0.34 to 0.38) | <0.001 | 0.42 (0.40 to 0.45) | <0.001 |
| G3b | 30,895 | 15.2% (14.6 to 15.8) | 7.1% (6.8 to 7.5) | 0.43 (0.40 to 0.46) | <0.001 | 0.48 (0.44 to 0.51) | <0.001 |
| G4 | 7029 | 25.5% (24.0 to 27.0) | 18.6% (17.4 to 19.9) | 0.66 (0.60 to 0.73) | <0.001 | 0.67 (0.61 to 0.75) | <0.001 |
| G5ND | 1383 | 32.6% (29.3 to 35.9) | 24.5% (21.1 to 28.0) | 0.71 (0.58 to 0.87) | 0.001 | 0.73 (0.60 to 0.90) | 0.003 |
| Albuminuriaa | 20,751 | 14.0% (13.4 to 14.6) | 8.6% (8.0 to 9.2) | 0.59 (0.54 to 0.64) | <0.001 | 0.60 (0.55 to 0.65) | <0.001 |
| Meeting KDIGO criteria for referralb | 26,853 | 18.2% (17.5 to 18.8) | 11.5% (11.0 to 12.0) | 0.61 (0.57 to 0.65) | <0.001 | 0.62 (0.58 to 0.66) | <0.001 |
| Meeting regional criteria for referralc | 17,022 | 17.2% (16.4 to 18.0) | 11.5% (10.8 to 12.2) | 0.65 (0.60 to 0.71) | <0.001 | 0.63 (0.57 to 0.68) | <0.001 |
| Previously referred | 4944 | 18.1% (16.6 to 19.7) | 13.9% (12.5 to 15.2) | 0.72 (0.63 to 0.83) | <0.001 | 0.85 (0.74 to 0.99) | 0.03 |
Risk of receiving a CKD diagnosis is among those without prior diagnosis. Of 227,847 included individuals, 11,328 had a history of CKD diagnosis. CVD, cardiovascular disease; KDIGO, Kidney Disease: Improving Global Outcomes; ND, non–dialysis dependent.
Albuminuria A2 and A3 combined.
Meeting KDIGO referral criterion of eGFR<30 ml/min per 1.73 m2, presence of albuminuria A3, or CKD and refractory hypertension.
Meeting regional referral criterion of age <50 years, albuminuria A3 and age 50–80 years, albumin-creatinine ratio >100 mg/mmol and age >80 years, or eGFR<30 ml/min per 1.73 m2 and age >80 years.
Adjusted for age at creatinine measurement, albuminuria category (no test, albuminuria A1, A2, A3), eGFR, history of referral, KDIGO criteria for referral, highest educational attainment, hypertension, diabetes, myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic obstructive pulmonary disease, and malignancy.
Figure 1.
Years 2009–2017 time trends in CKD care indicators among persons with first-ever eGFR<60 ml/min per 1.73 m2 by sex, using men in 2009 as reference. Although in general indicators of CKD care have improved over time, women with CKD have been persistently less likely to receive the same degree of recognition, referral to kidney specialist and monitoring of kidney function over time than men. Time trends in the risk of receiving (A) a diagnosis of CKD, (B) visiting a nephrologist, (C) remeasurement of creatinine, and (D) measurement of albuminuria in the next 18 months using men in 2009 as reference, by sex and year of inclusion, and at first occurrence of an eGFR measurement <60 ml/min per 1.73 m2 in the Stockholm region from 2009 to 2017. Hazard ratios are presented in Supplemental Table 5.
Visiting a Nephrologist
Among those who had not visited a nephrologist any time before baseline (n=217,341), women were less likely than men to visit a nephrologist in the next 18 months (unadjusted HR, 0.46; 95% CI, 0.43 to 0.48, Table 2). This difference was observed across all subgroups. Few people (11.4% of men and 6.8% of women) who had at least one KDIGO criteria for referral (Table 2) visited a nephrologist in the next 18 months, with a lower referral risk for women compared with men (unadjusted HR, 0.57; 95% CI, 0.52 to 0.63). Results were similar when evaluating single KDIGO referral criteria or regional recommendations (Supplemental Table 6). Multivariable adjustment slightly attenuated but did not abrogate these associations (Table 2). There have been modest improvements in referral patterns over time (Figure 1B, Supplemental Table 5), but women were persistently at lower risk of referral compared with men.
Table 2.
Cumulative incidence and hazard ratios of visiting a nephrologist in the next 18 months from first eGFR measurement <60 ml/min per 1.73 m2 among individuals with no history of nephrologist referral in the Stockholm region from 2009 to 2017, women compared with men
| Category | 18-Month Cumulative Incidence Estimates (95% CI) of Visiting a Nephrologist | HR (95% CI) of Visiting a Nephrologist in the Next 18 Months | |||||
|---|---|---|---|---|---|---|---|
| Number of Individuals | Men | Women | Unadjusted HR Women versus Men |
P Value | Adjusted HRd Women versus Men | P Value | |
| Overall | 217,341 | 4.2% (4.0–4.3) | 2.0% (1.9–2.0) | 0.46 (0.43–0.48) | <0.001 | 0.58 (0.55–0.61) | <0.001 |
| By age categories, yr | |||||||
| <65 | 42,531 | 7.7% (7.3–8.0) | 4.3% (4.0–4.6) | 0.54 (0.50–0.59) | <0.001 | 0.63 (0.58–0.68) | <0.001 |
| 65–75 | 61,359 | 3.9% (3.7–4.2) | 2.0% (1.9–2.2) | 0.50 (0.46–0.55) | <0.001 | 0.61 (0.56–0.68) | <0.001 |
| >75 | 113,451 | 2.6% (2.5–2.8) | 1.2% (1.–1.3) | 0.45 (0.42–0.50) | <0.001 | 0.49 (0.45–0.54) | <0.001 |
| By presence of comorbidities | |||||||
| Hypertension | 134,374 | 4.0% (3.9–4.2) | 1.9% (1.8–2.0) | 0.46 (0.43–0.49) | <0.001 | 0.61 (0.57–0.65) | <0.001 |
| Diabetes | 41,687 | 5.1% (4.8–5.4) | 2.9% (2.7–3.1) | 0.55 (0.50–0.61) | <0.001 | 0.69 (0.62–0.76) | <0.001 |
| CVD | 78,617 | 4.0% (3.8–4.2) | 1.9% (1.8–2.1) | 0.47 (0.43–0.51) | <0.001 | 0.59 (0.54–0.65) | <0.001 |
| By CKD category | |||||||
| G3a | 175,889 | 2.8% (2.7–2.9) | 1.2% (1.2–1.3) | 0.44 (0.41–0.47) | <0.001 | 0.54 (0.50–0.58) | <0.001 |
| G3b | 31,990 | 7.4% (6.9–7.8) | 2.2% (3.1–3.6) | 0.43 (0.39–0.47) | <0.001 | 0.58 (0.52–0.64) | <0.001 |
| G4 | 8012 | 16.4% (15.2–17.6) | 9.5% (8.6–10.4) | 0.53 (0.47–0.61) | <0.001 | 0.69 (0.60–0.78) | <0.001 |
| G5ND | 1450 | 29.4% (26.3–32.5) | 20.8% (17.7–24.0) | 0.67 (0.54–0.83) | <0.001 | 0.78 (0.63–0.97) | 0.03 |
| Albuminuriaa | 20,978 | 8.2% (7.7–8.7) | 5.7% (5.2–.2) | 0.68 (0.61–0.76) | <0.001 | 0.69 (0.62–0.77) | <0.001 |
| Meeting KDIGO criteria for referralb | 28,016 | 11.4% (10.9–11.9) | 6.8% (6.4–7.3) | 0.58 (0.53–0.63) | <0.001 | 0.68 (0.63–0.74) | <0.001 |
| Meeting regional criteria for referralc | 17,475 | 13.4% (12.7–14.1) | 7.9% (7.3–8.5) | 0.57 (0.52–0.63) | <0.001 | 0.63 (0.57–0.69) | <0.001 |
CVD, cardiovascular disease; ND, nondialysis dependent; KDIGO, Kidney Disease: Improving Global Outcomes.
Albuminuria A2 and A3 combined.
Meeting KDIGO referral criterion of eGFR <30 ml/min per 1.73 m2, presence of albuminuria A3, or CKD and refractory hypertension.
Meeting regional referral criterion of age <50 years, albuminuria A3 and age 50–80 years, albumin-creatinine ratio >100 mg/mmol and age >80 years, or eGFR <30 ml/min per 1.73 m2 and age >80 years.
Adjusted for age at creatinine measurement, albuminuria category (no test, albuminuria A1, A2, A3), eGFR, history of referral, KDIGO criteria for referral, highest educational attainment, hypertension, diabetes, myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic obstructive pulmonary disease, and malignancy.
Monitoring of Creatinine and Albumin
A high proportion of people (91.1% of men and 87.1% of women) had at least one remeasurement of creatinine taken in the next 18 months from inclusion (Table 3). The proportion of people who had an albuminuria measurement was lower (34.3% of men and 28.1% of women, Table 3). For the composite of both creatinine and albuminuria measurement, proportions were similar to those for albuminuria measurement (Supplemental Table 7). For each of these markers, men were measured earlier than women (Table 3 and Supplemental Table 7). This difference was observed across all subgroups. The sex differences were attenuated after multivariable adjustment, but persisted (e.g., remeasurement of creatinine in people with hypertension: unadjusted HR, 0.75; 95% CI, 0.75 to 0.76 and adjusted HR, 0.82; 95% CI, 0.81 to 0.83, Supplemental Table 8). Results were similar when evaluating single KDIGO referral criteria or regional recommendations (Supplemental Table 9). In recent years, remeasurement of creatinine was stable (Figure 1C), but time to albuminuria monitoring improved for both sexes (Figure 1D). In any case, monitoring of creatinine and albuminuria was persistently lower in women compared with men.
Table 3.
Cumulative incidence and hazard ratios of re-measurement creatinine or measurement of albuminuria in the next 18 months from first eGFR measurement <60 ml/min per 1.73 m2 in the Stockholm region from 2009 to 2017, women compared with men
| Category | Re-Measurement of Creatinine | Measurement of Albuminuria | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 18-Month Cumulative Incidence Estimates (95% CI) | HR (95% CI) | 18-Month Cumulative Incidence Estimates (95% CI) | HR (95% CI) | ||||||
| Number of Individuals | Men | Women | Unadjusted HR Women versus Men |
P Value | Men | Women | Unadjusted HR Women versus Men |
P Value | |
| Overall | 227,847 | 91.1% (90.0 to 91.3) | 87.1% (86.9 to 87.2) | 0.75 (0.74 to 0.76) | <0.001 | 34.3% (34.1 to 34.6) | 28.1% (27.9 to 28.4) | 0.76 (0.75 to 0.78) | <0.001 |
| By age categories, yr | |||||||||
| <65 | 46,772 | 90.0% (89.8 to 90.5) | 86.2% (85.7 to 86.6) | 0.79 (0.78 to 0.81) | <0.001 | 41.5% (40.9 to 42.1) | 36.9% (36.3 to 37.5) | 0.84 (0.82 to 0.87) | <0.001 |
| 65–75 | 63,983 | 92.2% (91.9 to 92.5) | 87.7% (87.3 to 88.0) | 0.72 (0.71 to 0.74) | <0.001 | 38.4% (37.9 to 39.0) | 33.3% (32.7 to 33.8) | 0.81 (0.79 to 0.83) | <0.001 |
| >75 | 117,092 | 90.8% (90.5 to 91.1) | 87.0% (86.8 to 87.3) | 0.76 (0.75 to 0.77) | <0.001 | 27.8% (27.4 to 28.2) | 23.0% (22.7 to 23.3) | 0.77 (0.76 to 0.79) | <0.001 |
| By presence of comorbidities | |||||||||
| Hypertension | 141,688 | 92.9% (92.7 to 93.1) | 89.6% (89.4 to 89.8) | 0.75 (0.75 to 0.76) | <0.001 | 38.2% (37.8 to 38.5) | 30.6% (30.0 to 30.9) | 0.74 (0.73 to 0.76) | <0.001 |
| Diabetes | 44,710 | 95.3% (95.1 to 95.6) | 93.5% (93.2 to 93.9) | 0.78 (0.77 to 0.80) | <0.001 | 55.1% (54.4 to 55.7) | 49.1% (48.4 to 49.7) | 0.82 (0.80 to 0.84) | <0.001 |
| CVD | 82,793 | 93.0% (92.8 to 93.3) | 90.1% (89.8 to 90.4) | 0.79 (0.78 to 0.80) | <0.001 | 32.7% (32.3 to 33.2) | 25.8% (25.3 to 26.2) | 0.74 (0.72 to 0.76) | <0.001 |
| By CKD category | |||||||||
| G3a | 181,540 | 90.5% (90.3 to 90.7) | 86.2% (86.0 to 86.4) | 0.75 (0.74 to 0.76) | <0.001 | 33.3% (32.9 to 33.6) | 27.9% (27.6 to 28.2) | 0.79 (0.78 to 0.80) | <0.001 |
| G3b | 34,207 | 93.1% (92.7 to 93.5) | 89.9% (89.5 to 90.4) | 0.73 (0.72 to 0.75) | <0.001 | 35.4% (34.7 to 36.2) | 27.1% (26.4 to 27.7) | 0.69 (0.66 to 0.72) | <0.001 |
| G4 | 9773 | 94.3% (93.6 to 94.9) | 90.9% (90.1 to 91.7) | 0.75 (0.72 to 0.78) | <0.001 | 44.0% (42.6 to 45.4) | 33.0% (31.7 to 34.3) | 0.66 (0.62 to 0.71) | <0.001 |
| G5ND | 2327 | 94.5% (93.1 to 95.6) | 94.3% (92.7 to 95.6) | 0.92 (0.85 to 1.00) | 0.063 | 53.4% (50.6 to 56.0) | 48.1% (45.0 to 51.2) | 0.83 (0.74 to 0.94) | 0.002 |
| Albuminuriaa | 24,996 | 94.9% (94.6 to 95.3) | 93.3% (92.8 to 93.7) | 0.84 (0.82 to 0.86) | <0.001 | 63.4% (62.6 to 64.2) | 58.4% (57.5 to 59.4) | 0.87 (0.84 to 0.91) | <0.001 |
| Meeting KDIGO criteria for referralb | 32,652 | 95.0% (94.7 to 95.3) | 92.8% (92.4 to 93.2) | 0.82 (0.81 to 0.84) | <0.001 | 50.5% (49.7 to 51.2) | 40.3% (39.5 to 41.1) | 0.73 (0.71 to 0.75) | <0.001 |
| Meeting regional criteria for referralc | 21,239 | 91.2% (90.7 to 91.7) | 88.6% (88.0 to 89.2) | 0.90 (0.87 to 0.93) | <0.001 | 50.5% (49.6 to 51.4) | 39.6% (38.6 to 40.6) | 0.73 (0.70 to 0.76) | <0.001 |
| Previously referred | 10,506 | 95.8% (95.2 to 96.3) | 94.8% (94.1 to 95.4) | 0.86 (0.83 to 0.90) | <0.001 | 63.7% (62.5 to 65.0) | 57.6% (56.2 to 59.0) | 0.83 (0.79 to 0.88) | <0.001 |
CVD, cardiovascular disease; KDIGO, Kidney Disease: Improving Global Outcomes; ND, non–dialysis dependent.
Albuminuria A2 and A3 combined.
Meeting KDIGO referral criterion of eGFR<30 ml/min per 1.73 m2, presence of albuminuria A3, or CKD and refractory hypertension.
Meeting regional referral criterion of age <50 years, albuminuria A3 and age 50–80 years, albumin-creatinine ratio >100 mg/mmol and age >80 years, or eGFR<30 ml/min per 1.73 m2 and age >80 years.
Pharmacological Management of CKD
Overall, a higher proportion of men were on RASi treatment (56% of men versus 47% of women) and statins (38% of men versus 29% of women). Although the use of these medications was higher in subgroups with guideline-recommended indications, the proportion of treated women was lower than the proportion of treated men (Table 4). Women had lower odds of receiving RASi; for instance, among patients with diabetes and albuminuria A2, the unadjusted OR comparing women to men was 0.72 (95% CI, 0.64 to 0.81, Table 4). In addition, women had lower odds of being on statin treatment. In trend analyses (Supplemental Figure 2, Supplemental Table 10), these differences of RASi and statin use persisted over time.
Table 4.
Differences in odds of being on RASi or statin treatment at first eGFR measurement <60 ml/min per 1.73 m2 in the Stockholm region from 2009 to 2017, women compared with men
| Category | Proportion of Patients Treated | Odds (95% CI) of Receiving Guideline-Recommended Medications | |||||
|---|---|---|---|---|---|---|---|
| Number of Individuals | Men | Women | Unadjusted OR Women versus Men |
P Value | Adjusted ORa Women versus Men |
P Value | |
| RASi | |||||||
| Overall | 227,847 | 56.2% | 46.7% | 0.68 (0.67 to 0.69) | <0.001 | 0.68 (0.66 to 0.69) | <0.001 |
| Among people with diabetes and albuminuria A2 | 7688 | 81.2% | 76.6% | 0.72 (0.64 to 0.81) | <0.001 | 0.68 (0.59 to 0.77) | <0.001 |
| Among people with albuminuria A3 | 8506 | 78.2% | 66.4% | 0.60 (0.54 to 0.65) | <0.001 | 0.68 (0.61 to 0.76) | <0.001 |
| Statins | |||||||
| Overall | 227,847 | 38.2% | 28.8% | 0.65 (0.64 to 0.67) | <0.001 | 0.78 (0.76 to 0.79) | <0.001 |
| Among people aged ≥50 yrs | 217,384 | 39.6% | 29.7% | 0.64 (0.63 to 0.66) | <0.001 | 0.80 (0.78 to 0.82) | <0.001 |
| Among people aged 18–49 yr with | |||||||
| Coronary disease | 166 | 74.6% | 60.4% | 0.54 (0.27 to 1.09) | 0.09 | 0.41 (0.15 to 1.12) | 0.08 |
| Diabetes | 939 | 45.1% | 34.1% | 0.63 (0.48 to 0.82) | 0.001 | 0.74 (0.55 to 0.99) | 0.05 |
| Prior ischemic stroke | 174 | 46.4% | 33.9% | 0.62 (0.33 to 1.17) | 0.14 | 0.62 (0.27 to 1.42) | 0.26 |
Adjusted for age at creatinine measurement, albuminuria category (no test, albuminuria A1, A2, A3), eGFR, history of referral, KDIGO, Kidney Disease: Improving Global Outcomes (KDIGO) criteria for referral, highest educational attainment, hypertension, diabetes, myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic obstructive pulmonary disease, and malignancy.
Supporting Analyses
In total, 5906 men (6%) and 4600 women (4%) visited a nephrologist before the index date. Among people undergoing remeasurement of creatinine, 80,761 had another eGFR<60 ml/min per 1.73 m2 between 3 and 18 months after the index date (i.e., had confirmed CKD), on the basis of the first available creatinine measurement in that time period. This was observed in 46% of retested men and 42% of retested women. In both these subgroups (people referred or with confirmed CKD), the performance of nephrology care indicators was slightly better than that observed in the cohort overall, but sex differences persisted throughout most strata (Tables 1 and 3, Supplemental Tables 4, 11, 12 and 13). Results were also similar when using the Lund–Malmö eGFR equation (Supplemental Table 14), when additionally adjusting for creatinine levels (Supplemental Table 15), and before/after the implementation of automatic eGFR reporting (Supplemental Table 16); in all these analyses, we observed some attenuation in the differences observed, but differences remained.
Discussion
In this study of people with probable CKD, we observed profound sex differences in detection, recognition, and monitoring that persisted over time: women were less likely to receive a CKD diagnosis, to visit a nephrologist, to have their creatinine and albuminuria measured, or to receive guideline-recommended therapies. These differences were similarly observed among high-risk groups, among people with evidence-based indications for medications, and among people with confirmed CKD at retesting.
We are not aware of other studies focusing on this issue in the general population. Administrative databases are culturally bounded, and we recognize this is a study of practices in Stockholm between 2009 and 2017. We do not know whether our findings are generalizable until these issues are assessed in other health systems. However, we have found isolated observations, often anecdotal or reported as secondary findings in reports from the United Kingdom,5,21,22,32 Canada,23,33 and the United States,24,25,34–36 that support the existence of such sex gaps. We present unadjusted data because the variable “sex” cannot be confounded (i.e., other biologic or social factors do not exert causal influence on a person’s sex). We recognize, however, that the effects of sex or gender on outcomes can be mediated by individual and societal structural biases that our study could not account for—for example, if individual women are less likely to seek referral or if clinics are less likely to accept referrals of women. We explored multivariable adjustment to examine whether differences in comorbidity explained the differences observed. In general, these analyses showed some attenuation of the effect, suggesting that appropriate factors are being taken into consideration in clinical decisions. However, adjustment did not abrogate the sex differences reported.
Issuing a diagnostic code is relevant beyond reimbursement purposes. Previous studies show that among persons with objective laboratory evidence for CKD, those with a diagnostic code were more likely to achieve quality of nephrology care indicators22 and less likely to receive nephrotoxic medications.37 Monitoring of eGFR and albuminuria in people at risk is recommended by major guidelines1 because of the opportunity to implement therapies that slow kidney damage and reduce cardiovascular risk.38 RASi decreases albuminuria, reduces the rate of eGFR decline,39–41 reduces ESKD in people with albuminuria,42 and reduces cardiovascular outcomes.43 Statins reduce cardiovascular events.44 All these indicators and medications scored worse for women, through all albuminuria categories and among people with indications for treatment, monitoring, and diagnosis.
We observed high rates of creatinine testing, which contrasted with low rates of albuminuria testing, but in both cases, women were disadvantaged. We speculate that creatinine is measured routinely for many indications, and although this information is stated in the health care records, it may not be interpreted as a marker of kidney disease that requires evaluation and follow-up, especially in older adults. Interpreting creatinine as an indicator of kidney function may be problematic: at the same level of creatinine, men have better kidney function than women because of higher muscle mass on average and increased endogenous creatinine production.45 To some extent, this disparity is mitigated by the use of eGFR, which includes a term for sex and produces output that is adjusted for body size. Because physicians may lack the time to calculate eGFR, automatic eGFR reporting has been implemented in many health systems to facilitate CKD detection and monitoring.46 We did observe some narrowing in the sex gap after adjusting for creatinine, or after the implementation of automatic eGFR reporting in our health system. However, differences persisted.
Among those who satisfied guideline-recommended criteria for nephrologist referral, fewer women than men visited a nephrologist, even among those who had CKD confirmed with repeated testing. Late referral to nephrology units has been associated with faster disease progression and worse outcomes,47 and in our data sex-related differences in referral patterns, although somewhat attenuated with increasing severity, were large. It has been argued that nephrology units lack the capacity to accommodate so many patients with CKD, and that management is best decentralized, with larger responsibility for primary health care settings. This is the case in Sweden, and in this regard, we note that our study can only quantify accepted referrals that lead to a visit. This may be only a portion of all referrals, because of referral triage by nephrologists, and nonadherence by patients. Nephrologists may also give advice to the general practitioner regarding monitoring of kidney function or implementation of therapies, or evaluate the condition with all available data, including imaging, without necessarily physically seeing the patient. However, whether this advice took place, women meeting objective criteria for referral in terms of albuminuria and eGFR level were still disadvantaged. This suggests that some of the previously observed differences in the proportion of men and women at referral might reflect negative selection of women for referral by primary health care providers. To mitigate the possibility of lack of resources in nephrology, to risk-stratify individuals and identify those at high risk for progressive disease, and to increase the appropriate prescribing of RASi, it is essential that albuminuria testing is more widely undertaken, in both men and women.
We observed that the disadvantage to women was less pronounced among people with more severe CKD and it affected prescribing perhaps less than it affected recognition, remeasurement, and referral. It is possible that stronger indications for recognition and action may partially overcome whatever biases lead to under-recognition and undertreatment. However, large effects persisted: for example, women with G5ND CKD were 23% less likely than men to see a nephrologist during follow-up. We think it is unlikely this reflects competing risk of death, which has been reported to be lower in women with CKD compared with men.48 The finding of a lower proportion of women starting dialysis in a given time period or the finding of lower GFR at initiation in women48 may reflect increased time-to-event rather than fewer lifetime events. More likely these strong and pervasive effects are social: they demand further study and mitigation.
A strength of our study is the complete health care coverage and richness of information from a region with universal tax-funded health care. This may minimize health care access bias, which in nonsubsidized health systems has been shown to negatively affect women with CKD.35,36 Our study also has limitations. By identifying CKD from a single eGFR test we may have misclassified some patients. However, requiring a confirmatory eGFR would have invalidated our evaluation of laboratory monitoring rates. Furthermore, our findings were similar in those with confirmed CKD. Because we measured completed actions—a nephrology visit completed, laboratory tests performed, and prescriptions filled—it is possible that sex differences in adherence led to some of the observed differences. A Swedish survey suggests that for prescription medications, men are more likely than women to forget to pick up a prescription, and women are more likely than men to pick up a prescription, but not take the medication.49 If so, true differences might be larger than we estimated. However, the outcome “diagnosis of CKD” is not subject to any adherence effect and shows a strong effect of sex. Finally, we are unable to explain these effects beyond conscious and unconscious bias on the part of health care professionals.
To conclude, this study observes profound sex differences in recognition, specialist care access, monitoring and management of CKD that are not explained by differences in comorbidity. We believe that efforts to improve this and ensure equitable care between sexes could have important implications for justice and could reduce the burden of CKD.
Disclosures
C.M. Clase reports receiving payment for lectures, advisory boards, or study grants unrelated to this study from Amgen, Astellas, AstraZeneca, Baxter, Boehringer-Ingelheim, Janssen, Leo Pharma, Pfizer, and Sanofi; reports consultancy agreements with Amgen, Astellas, Baxter, Boehringer-Ingelheim, Leo Pharma, Janssen, Ministry of Health Ontario, and Pfizer; reports receiving research funding for work on the ONTARGET and TRANSCEND databases; these trials were funded by Boehringer-Ingelheim, coinvestigator on the REPORT study, funded by Astellas, and on the FLUID study, funded by Baxter; reports receiving honoraria from Astellas, Janssen, Ontario Ministry of Health, Sanofi, and the University of Alberta; and reports having an advisory or leadership role as ACP journal club associate editor, Canadian Journal of Kidney Health and Disease editor-in-chief, co-chair of a KDIGO potassium controversies conference sponsored at arm's length by Fresenius Medical Care in 2018, Bayer HealthCare, Relypsa, and Vifor Fresenius Medical Care, which had a direct financial interest in the outcome of the conference. E.L. Fu reports receiving a support by a Rubicon Grant of the Netherlands. M. Evans reports receiving payment for lectures, advisory boards, or study grants unrelated to this study from Astellas, AstraZeneca, Fresenius Medical Care, Baxter, and Vifor Pharma; reports receiving research funding from an Astellas Pharma institutional grant; reports receiving honoraria from a payment for lectures by Astellas, AstraZeneca, Baxter Healthcare, Fresenius Medical Care, Vifor Pharma; reports having an advisory or leadership role with Astellas, AstraZeneca, and the Vifor Pharma Advisory board; and reports having other interests or relationships as a Member of the Steering committee in the Swedish Renal Registry and the European Renal Association (ERA) Registry Committee. J.J. Carrero reports receiving support from the Swedish Heart and Lung Foundation, and the Westman Foundation; reports receiving payment for lectures, advisory boards, or study grants unrelated to this study from Abbott, Astellas, AstraZeneca, Baxter, Bayer, Fresenius Medical Care, Fresenius Kabi, and Vifor Pharma; reports having consultancy agreements with AstraZeneca, Bayer, and Nestlé; reports receiving research funding from Amgen, Astellas, AstraZeneca, the Swedish Research Council, Swedish Heart and Lung Foundation, and ViforPharma; reports having an advisory or leadership role on the Advisory Committee of Nestlé, the Editorial board of the American Journal of Kidney Disease, European Heart Journal, Journal of Nephrology, and Nephrology, Dialysis and Transplantation; and reports having other interests or relationships with the European Renal Nutrition working group at the ERA-EDTA, and International Society of Renal Nutrition and Metabolism. M. Hecking reports receiving research funding from Astellas Pharma, Boehringer-Ingelheim, Eli Lilly, and Siemens Healthcare; and reports receiving support from the Austrian Science Fund (#KL754-B). O. Swartling reports receiving support from the Clinical Scientist Training Program and research internship at Karolinska Institutet. All remaining authors have nothing to disclose.
Funding
This work is supported by the Swedish Research Council (2019-01059).
Supplementary Material
Acknowledgment
Because E. Fu is an editorial fellow for the JASN, he was not involved in the peer review process for this manuscript; a guest editor oversaw the peer review and decision-making process for this manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Sex Disparities in the Quality of Care for CKD,” on pages 1804–1806.
Author Contributions
J.J. Carrero, C.M. Clase, M. Evans, E.L. Fu, M. Hecking, S. Hödlmoser, O. Swartling, and Y. Trolle-Lagerros conceptualized the study; J.J. Carrero, O. Swartling, and Y. Yang were responsible for the data curation; O. Swartling was responsible for the formal analysis; J.J. Carrero was responsible for the funding acquisition, investigation, methodology, and project administration; J.J. Carrero, M. Evans, and Y. Trolle-Lagerros provided supervision; O. Swartling wrote the original draft; and J.J. Carrero, C.M. Clase, M. Evans, E.L. Fu, M. Hecking, S. Hödlmoser, Y. Trolle-Lagerros, and Y. Yang reviewed and edited the manuscript.
Data Sharing Statement
Data will be available for collaborative research under reasonable request and fulfillment of GDPR regulations. For inquiries, please send your proposal to the Steering Committee of the SCREAM project (Email: juan.jesus.carrero@ki.se).
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2022030373/-/DCSupplemental.
Supplemental Table 1. Definition of comorbidities using ICD-10 codes and medications using anatomical therapeutic chemical codes.
Supplemental Table 2. Baseline characteristics for health care users at first eGFR measurement <60 ml/min per 1.73 m2 in the Stockholm region from 2009 to 2017, overall and stratified by sex.
Supplemental Table 3. Baseline characteristics of health care users at first occurrence of an eGFR measurement <60 ml/min per 1.73 m2 each year, by year of inclusion, in the Stockholm region from 2009 to 2017.
Supplemental Table 4. Odds ratios of having received a CKD diagnosis by sex at first eGFR measurement <60 ml/min per 1.73 m2 in the Stockholm region from 2009 to 2017.
Supplemental Table 5. Risk of receiving a diagnosis of CKD, visiting a nephrologist, remeasurement of creatinine and measurement of albuminuria in the next 18 months using men in 2009 as reference, by sex and year of inclusion and at first occurrence of an eGFR measurement <60 ml/min per 1.73 m2 in the Stockholm region from 2009 to 2017.
Supplemental Table 6. HRs of receiving a diagnosis of CKD and visiting a nephrologist in the next 18 months from first eGFR measurement <60 ml/min per 1.73 m2 in the Stockholm region from 2009 to 2017, by KDIGO and regional criteria for referral, comparing women to men.
Supplemental Table 7. Cumulative incidence and HRs of remeasurement of creatinine and measurement of albuminuria (both metrics together) in the next 18 months from first eGFR measurement <60 ml/min per 1.73 m2 in the Stockholm region from 2009 to 2017, women compared with men.
Supplemental Table 8. Risk of remeasurement of creatinine or measurement of albuminuria in the next 18 months from first eGFR measurement <60 ml/min per 1.73 m2 in the Stockholm region from 2009 to 2017, women compared with men.
Supplemental Table 9. Hazard ratios of remeasurement of creatinine and measurement of albuminuria in the next 18 months from first eGFR measurement <60 ml/min per 1.73 m2 in the Stockholm region from 2009 to 2017, by KDIGO and regional criteria for referral, comparing women to men.
Supplemental Table 10. Unadjusted OR of current RASi and statin treatment using men in 2009 as reference, by sex and year of inclusion.
Supplemental Table 11. Hazard ratios of receiving a CKD diagnosis and visiting a nephrologist in the next 18 months among patients with two consecutive measurements of eGFR<60 ml/min per 1.73 m2 (i.e., confirmed CKD), women compared with men.
Supplemental Table 12. Hazard ratios of remeasurement of creatinine or measurement of albuminuria in the next 18 months among patients with two consecutive measurements of eGFR<60 ml/min per 1.73 m2 (i.e., confirmed CKD), women compared with men.
Supplemental Table 13. Differences in odds of being on renin-angiotensin system inhibitor treatment or statin treatment among people with a recorded visit to a nephrologist any time before their first eGFR measurement <60 ml/min per 1.73 m2 in the Stockholm region from 2009 to 2017, women compared with men.
Supplemental Table 14. Study outcomes among men and women with probable CKD (first-encountered eGFR measurement <60 ml/min per 1.73 m2) in the Stockholm region during 2015 to 2017, using the Lund–Malmö eGFR equation.
Supplemental Table 15. Study outcomes among men and women with probable CKD (first-encountered eGFR measurement <60 ml/min per 1.73 m2) in the Stockholm region during 2009 to 2017, additionally adjusted for serum/plasma creatinine.
Supplemental Table 16. Study outcomes among men and women with probable CKD (first-encountered eGFR measurement <60 ml/min per 1.73 m2) in the Stockholm region during 2009 to 2017, evaluating two distinct periods (before or after automatic eGFR reporting) with P value for interaction terms.
Supplemental Figure 1. Patient flow chart into the study.
Supplemental Figure 2. Time trends in the odds of being on RASi or statin treatment, between men and women during the period 2009–2017. In each year, the first encountered eGFR<60 ml/min per 1.73 m2 was considered the cohort baseline. Men in 2009 were selected as the reference category. The ORs are presented in Supplemental Table 10.
References
- 1.Kidney Disease : Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Available at: https://kdigo.org/guidelines/ckd-evaluation-and-management/. Accessed January 23, 2021
- 2.GBD Chronic Kidney Disease Collaboration : Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 395: 709–733, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park JI, Baek H, Jung HH: CKD and health-related quality of life: The Korea national health and nutrition examination survey. Am J Kidney Dis 67: 851–860, 2016 [DOI] [PubMed] [Google Scholar]
- 4.Legrand K, Speyer E, Stengel B, Frimat L, Ngueyon Sime W, Massy ZA, et al. : Perceived health and quality of life in patients with CKD, including those with kidney failure: Findings from national surveys in France. Am J Kidney Dis 75: 868–878, 2020 [DOI] [PubMed] [Google Scholar]
- 5.Jameson K, Jick S, Hagberg KW, Ambegaonkar B, Giles A, O’Donoghue D: Prevalence and management of chronic kidney disease in primary care patients in the UK. Int J Clin Pract 68: 1110–1121, 2014 [DOI] [PubMed] [Google Scholar]
- 6.Carrero JJ, Hecking M, Chesnaye NC, Jager KJ: Sex and gender disparities in the epidemiology and outcomes of chronic kidney disease. Nat Rev Nephrol 14: 151–164, 2018 [DOI] [PubMed] [Google Scholar]
- 7.Grams ME, Chow EK, Segev DL, Coresh J: Lifetime incidence of CKD stages 3-5 in the United States. Am J Kidney Dis 62: 245–252, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turin TC, Tonelli M, Manns BJ, Ahmed SB, Ravani P, James M, et al. : Lifetime risk of ESRD. J Am Soc Nephrol 23: 1569–1578, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hecking M, Bieber BA, Ethier J, Kautzky-Willer A, Sunder-Plassmann G, Säemann MD, et al. : Sex-specific differences in hemodialysis prevalence and practices and the male-to-female mortality rate: The Dialysis Outcomes and Practice Patterns Study (DOPPS). PLoS Med 11: e1001750, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Antlanger M, Noordzij M, van de Luijtgaarden M, Carrero JJ, Palsson R, Finne P, et al. : ERA-EDTA Registry: Sex differences in kidney replacement therapy initiation and maintenance. Clin J Am Soc Nephrol 14: 1616–1625, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Minutolo R, Gabbai FB, Chiodini P, Provenzano M, Borrelli S, Garofalo C, et al. : Collaborative Study Group on the Conservative Treatment of CKD of the Italian Society of Nephrology: Sex differences in the progression of CKD among older patients: Pooled analysis of 4 cohort studies. Am J Kidney Dis 75: 30–38, 2020 [DOI] [PubMed] [Google Scholar]
- 12.Swartling O, Rydell H, Stendahl M, Segelmark M, Trolle Lagerros Y, Evans M: CKD progression and mortality among men and women: A nationwide study in Sweden. Am J Kidney Dis 78: 190–199, 2021 [DOI] [PubMed] [Google Scholar]
- 13.Ricardo AC, Yang W, Sha D, Appel LJ, Chen J, Krousel-Wood M, et al. : CRIC Investigators: Sex-related disparities in CKD progression. J Am Soc Nephrol 30: 137–146, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morton RL, Turner RM, Howard K, Snelling P, Webster AC: Patients who plan for conservative care rather than dialysis: A national observational study in Australia. Am J Kidney Dis 59: 419–427, 2012 [DOI] [PubMed] [Google Scholar]
- 15.Tomlinson LA, Clase CM: Sex and the incidence and prevalence of kidney disease. Clin J Am Soc Nephrol 14: 1557–1559, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adams SV, Rivara M, Streja E, Cheung AK, Arah OA, Kalantar-Zadeh K, et al. : Sex differences in hospitalizations with maintenance hemodialysis. J Am Soc Nephrol 28: 2721–2728, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yan G, Norris KC, Greene T, Yu AJ, Ma JZ, Yu W, et al. : Race/ethnicity, age, and risk of hospital admission and length of stay during the first year of maintenance hemodialysis. Clin J Am Soc Nephrol 9: 1402–1409, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De La Mata NL, Rosales B, MacLeod G, Kelly PJ, Masson P, Morton RL, et al. : Sex differences in mortality among binational cohort of people with chronic kidney disease: Population based data linkage study. BMJ 375: e068247, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kleindorfer DO, Towfighi A, Chaturvedi S, Cockroft KM, Gutierrez J, Lombardi-Hill D, et al. : 2021 guideline for the prevention of stroke in patients with stroke and transient ischemic attack: A guideline from the American Heart Association/American Stroke Association. Stroke 52: e364–e467, 2021 [DOI] [PubMed] [Google Scholar]
- 20.American Diabetes Association : 3. Prevention or delay of type 2 diabetes: Standards of medical care in diabetes-2021. Diabetes Care 44[Suppl 1]: S34–S39, 2021 [DOI] [PubMed] [Google Scholar]
- 21.John R, Webb M, Young A, Stevens PE: Unreferred chronic kidney disease: A longitudinal study. Am J Kidney Dis 43: 825–835, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Kim LG, Cleary F, Wheeler DC, Caplin B, Nitsch D, Hull SA; UK National Chronic Kidney Disease Audit : How do primary care doctors in England and Wales code and manage people with chronic kidney disease? Results from the National Chronic Kidney Disease Audit. Nephrol Dial Transplant 33: 1373–1379, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bello AK, Ronksley PE, Tangri N, Kurzawa J, Osman MA, Singer A, et al. : Quality of chronic kidney disease management in Canadian primary care. JAMA Netw Open 2: e1910704, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hödlmoser S, Winkelmayer WC, Zee J, Pecoits-Filho R, Pisoni RL, Port FK, et al. : Sex differences in chronic kidney disease awareness among US adults, 1999 to 2018. PLoS One 15: e0243431, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qiao Y, Shin JI, Chen TK, Sang Y, Coresh J, Vassalotti JA, et al. : Association of albuminuria levels with the prescription of renin-angiotensin system blockade. Hypertension 76: 1762–1768, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carrero JJ, Elinder CG: The Stockholm CREAtinine Measurements (SCREAM) project: Fostering improvements in chronic kidney disease care. J Intern Med 291: 254–268, 2022 [DOI] [PubMed] [Google Scholar]
- 27.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. : CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration): A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.EQUALIS : Available at: https://equalis.se/en/. Accessed May 30, 2022.
- 29.Laugesen K, Ludvigsson JF, Schmidt M, Gissler M, Valdimarsdottir UA, Lunde A, et al. : Nordic health registry-based research: A review of health care systems and key registries. Clin Epidemiol 13: 533–554, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ludvigsson JF, Svedberg P, Olén O, Bruze G, Neovius M: The longitudinal integrated database for health insurance and labour market studies (LISA) and its use in medical research. Eur J Epidemiol 34: 423–437, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nyman U, Grubb A, Larsson A, Hansson LO, Flodin M, Nordin G, et al. : The revised Lund-Malmö GFR estimating equation outperforms MDRD and CKD-EPI across GFR, age and BMI intervals in a large Swedish population. Clin Chem Lab Med 52: 815–824, 2014 [DOI] [PubMed] [Google Scholar]
- 32.Fraser SD, Parkes J, Culliford D, Santer M, Roderick PJ: Timeliness in chronic kidney disease and albuminuria identification: A retrospective cohort study. BMC Fam Pract 16: 18, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manns L, Scott-Douglas N, Tonelli M, Weaver R, Tam-Tham H, Chong C, et al. : A population-based analysis of quality indicators in CKD. Clin J Am Soc Nephrol 12: 727–733, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stevens LA, Fares G, Fleming J, Martin D, Murthy K, Qiu J, et al. : Low rates of testing and diagnostic codes usage in a commercial clinical laboratory: Evidence for lack of physician awareness of chronic kidney disease. J Am Soc Nephrol 16: 2439–2448, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Xue JL, Eggers PW, Agodoa LY, Foley RN, Collins AJ: Longitudinal study of racial and ethnic differences in developing end-stage renal disease among aged Medicare beneficiaries. J Am Soc Nephrol 18: 1299–1306, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Kausz AT, Obrador GT, Arora P, Ruthazer R, Levey AS, Pereira BJG: Late initiation of dialysis among women and ethnic minorities in the United States. J Am Soc Nephrol 11: 2351–2357, 2000 [DOI] [PubMed] [Google Scholar]
- 37.Bosi A, Xu Y, Gasparini A, Wettermark B, Barany P, Bellocco R, et al. : Use of nephrotoxic medications in adults with chronic kidney disease in Swedish and US routine care. Clin Kidney J 15: 442–451, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luyckx VA, Cherney DZI, Bello AK: Preventing CKD in developed countries. Kidney Int Rep 5: 263–277, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jafar TH, Schmid CH, Levey AS: Effect of angiotensin-converting enzyme inhibitors on progression of nondiabetic renal disease. Ann Intern Med 137: 298–299, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD; The Collaborative Study Group : The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. N Engl J Med 329: 1456–1462, 1993 [DOI] [PubMed] [Google Scholar]
- 41.Cherney DZ, Perkins BA, Soleymanlou N, Maione M, Lai V, Lee A, et al. : Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation 129: 587–597, 2014 [DOI] [PubMed] [Google Scholar]
- 42.Jafar TH, Schmid CH, Landa M, Giatras I, Toto R, Remuzzi G, et al. : Angiotensin-converting enzyme inhibitors and progression of nondiabetic renal disease. A meta-analysis of patient-level data. Ann Intern Med 135: 73–87, 2001 [DOI] [PubMed] [Google Scholar]
- 43.Yusuf S, Teo KK, Pogue J, Dyal L, Copland I, Schumacher H, et al. : ONTARGET Investigators: Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 358: 1547–1559, 2008 [DOI] [PubMed] [Google Scholar]
- 44.Sharp Collaborative Group : Study of Heart and Renal Protection (SHARP): Randomized trial to assess the effects of lowering low-density lipoprotein cholesterol among 9,438 patients with chronic kidney disease. Am Heart J 160: 785–794, 2010 [DOI] [PubMed] [Google Scholar]
- 45.Swedko PJ, Clark HD, Paramsothy K, Akbari A: Serum creatinine is an inadequate screening test for renal failure in elderly patients. Arch Intern Med 163: 356–360, 2003 [DOI] [PubMed] [Google Scholar]
- 46.Foote C, Clayton PA, Johnson DW, Jardine M, Snelling P, Cass A: Impact of estimated GFR reporting on late referral rates and practice patterns for end-stage kidney disease patients: A multilevel logistic regression analysis using the Australia and New Zealand Dialysis and Transplant Registry (ANZDATA). Am J Kidney Dis 64: 359–366, 2014 [DOI] [PubMed] [Google Scholar]
- 47.Smart NA, Dieberg G, Ladhani M, Titus T: Early referral to specialist nephrology services for preventing the progression to end-stage kidney disease. Cochrane Database Syst Rev (6): CD007333, 2014 [DOI] [PubMed] [Google Scholar]
- 48.Hecking M, Tu C, Zee J, Bieber B, Hödlmoser S, Reichel H, et al. : Sex-specific differences in mortality and incident dialysis in the chronic kidney disease outcomes and practice patterns study. Kidney Int Rep 7: 410–423, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thunander Sundbom L, Bingefors K: Women and men report different behaviours in, and reasons for medication non-adherence: A nationwide Swedish survey. Pharm Pract (Granada) 10: 207–221, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.