Loss of kidney function disrupts normal calcium and phosphate homeostasis, and triggers increases in circulating concentrations of fibroblast growth factor 23 (FGF23) and parathyroid hormone (PTH). These adaptations help to maintain normal serum calcium and phosphate, but as CKD progresses to ESKD, they may also exert maladaptive effects (1). Secondary hyperparathyroidism contributes to bone disease in CKD (2), and FGF23- and PTH-mediated increases in per-nephron excretion of phosphate may accelerate progression to ESKD by exacerbating tubular injury (3). Excess FGF23 may contribute mechanistically to development of pathologic left ventricular hypertrophy (4), which is a cardiac substrate that underlies heart failure with preserved ejection fraction, atrial fibrillation, and death in CKD, all of which are associated with elevated FGF23 in prospective observational studies (5–7).
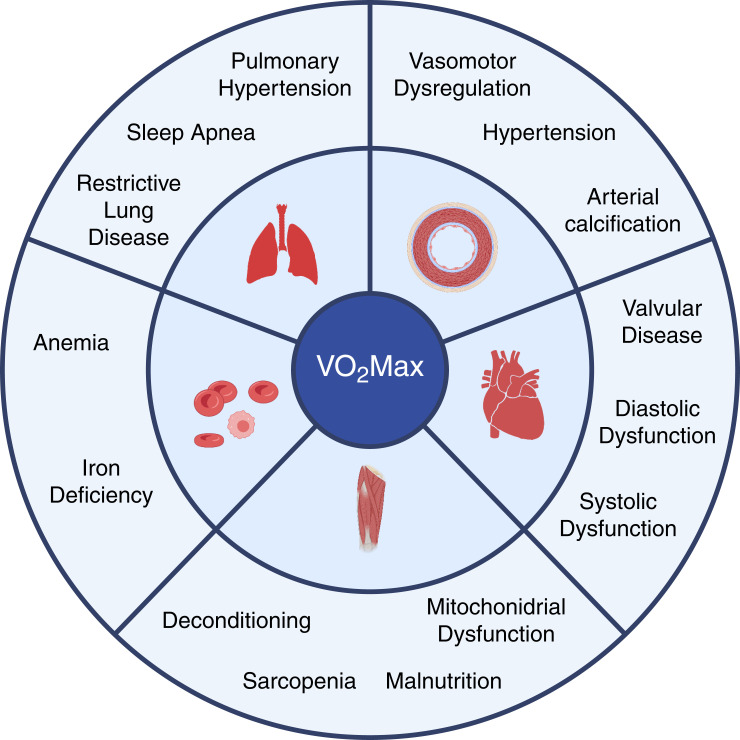
Between the molecular effects of FGF23 on cardiac myocytes and the epidemiologic data linking FGF23 excess to adverse cardiovascular outcomes lies an important gap: there are limited data on the effects of FGF23 on cardiovascular function in CKD. In this edition of Kidney360, Halim et al. (8) fill this gap by performing a secondary analysis of the Cardiopulmonary Exercise Testing in Renal Failure and After Kidney Transplantation (CAPER) Study, which used echocardiography and cardiopulmonary exercise testing (CPET) to investigate exercise capacity in patients with CKD 2 weeks before and 2 months and 1 year after kidney transplantation (9). Among the many parameters measured by CPET, VO2 Max was the primary outcome of the CAPER Study. VO2 Max quantifies total body oxygen consumption at maximum exercise. It provides a single integrated measurement that encompasses the contributions of all organs involved in exercise, including the heart, lungs, vessels, blood, and muscles. Diseases in any of these organs can reduce VO2 Max (Figure 1), and higher VO2 Max is associated with improved outcomes in a variety of clinical settings (10).
Figure 1.
Potential mechanisms of impaired VO2 Max in CKD that may be reversed by kidney transplantation. Cardiopulmonary exercise testing enables measurement of VO2 Max, which is defined as total body oxygen consumption at maximum exercise. VO2 Max is an integrated measure that encompasses the contributions of all systems involved in exercise. Diseases of the cardiovascular, respiratory, vascular, hematologic, and musculoskeletal systems can each reduce VO2 Max. CKD causes multiple alterations to each system that can be mitigated or reversed by kidney transplantation. How FGF23 excess might contribute to reduced VO2 Max in CKD requires further study. (Created with BioRender.com.)
The main findings of the CAPER Study were that VO2 Max was impaired in ESKD versus hypertensive controls without CKD, and significantly improved by 1 year post transplant compared with patients with ESKD who were cleared for transplant but remained on the waiting list. Interestingly, the functional improvement in VO2 Max and other exercise parameters occurred despite no structural benefit of transplantation on left ventricular mass index.
In their secondary analysis of the CAPER Study, Halim et al. (8) evaluated the association of FGF23 with exercise capacity at baseline and after kidney transplantation. The authors measured serial FGF23 at each time point when CPET was performed in 64 patients who underwent transplantation, 85 waitlisted nontransplanted patients with ESKD, and 83 hypertensive controls. It is not clear from the manuscript what accounted for the modest attrition of patients in the FGF23 substudy relative to the parent CAPER Study.
In cross-sectional analyses of patients at their baseline CPET assessments, higher FGF23 associated with reduced VO2 Max and impairments in several other markers of exercise capacity. These important new data associating higher FGF23 with impaired functional capacity have not been previously reported in patients with advanced CKD. They complement similar results from a study that linked FGF23 excess to impaired exercise capacity in a heart failure population that included a subgroup of patients with moderately reduced kidney function (11). Using echocardiography, the authors confirmed previously reported associations of FGF23 excess with higher left ventricular mass index, lower left ventricular ejection fraction, and higher left ventricular filling pressure (4).
In their longitudinal analyses, Halim et al. demonstrate that kidney transplantation progressively decreased FGF23 by two- and 12-months post transplant, which is consistent with prior studies (12). The authors report that transplantation simultaneously improved VO2 Max and multiple other exercise parameters but did not alter left ventricular mass index, as reported in the parent CAPER Study. Furthermore, the authors report that the longitudinal change in FGF23 associated with the longitudinal improvement in VO2 Max post transplant, but not with the parallel change over time in left ventricular mass index. The authors conclude that reductions of FGF23 may be a predictor of improvements in cardiovascular functional capacity.
A challenge of observational studies is discerning association versus causation. This issue is particularly fraught in the case of the association of change in FGF23 with change in VO2 Max after kidney transplantation, which simultaneously mitigates or reverses many complications of CKD. These include FGF23 excess and reduced VO2 Max, as shown by the authors, but also numerous other factors that may influence exercise capacity. For example, transplantation improves or corrects BP and vasomotor tone, volume status, sleep disorders, pulmonary V/Q mismatch, suppressed appetite, malnutrition, anemia, iron deficiency, and sarcopenia, among many other effects of uremia (Figure 1).
When assessing potential causality in observational studies, one important step is to demonstrate statistical significance of the relationship between exposure and outcome independently of potential confounders using multivariable analyses. In the current report, the authors appropriately used linear mixed models for repeated measures as the basis of their conclusions relating changes in FGF23 to changes in exercise capacity post transplantation. However, mixed models of a repeated measures outcome (in this case, VO2 Max) typically include multiple terms, not all of which were included in the current analyses. Random-effects terms are included to account for the correlation of repeated measures within individual patients chosen for inclusion in the study. Fixed-effects terms account for groups of patients exposed to different interventions, for example transplantation versus waitlisted. A time variable accounts for repeated time points. Interactions between fixed group and time account for the effect of the intervention over time, and covariates and their interactions with time account for potential confounders and their changes over time after the intervention.
In the current study, transplantation should have been included as a fixed-effect term (along with its interactions with time, FGF23, and covariates) because it was the key intervention during follow-up and was known to have a powerful effect on longitudinal improvements in VO2 Max. It was also the factor used to recruit and stratify the study population. Likewise, changes in other key covariates probably should have been included. For example, the authors appropriately adjusted for baseline hemoglobin and albumin, but not for their changes over time post transplant. Indeed, FGF23 was the only variable for which longitudinal change over time was modeled. For other potential confounders, such as eGFR, both baseline and change over time post transplant probably should have been included in the modeling. By not accounting for transplantation or post-transplant changes in other covariates that could influence VO2 Max, the model building strategy likely enabled misattribution of effects on VO2 Max to FGF23 rather than to transplantation itself or other changing covariates.
As a result, it is difficult to conclude whether FGF23 reduction post transplant is independently associated with improved VO2 Max or whether the observed association of FGF23 is an epiphenomenon of the effects of transplantation on other factors.
Despite these limitations, Halim et al. provide further evidence that support adverse effects of FGF23 on cardiac structure, and they advance the field by demonstrating associations of FGF23 excess with impaired exercise capacity in patients with advanced CKD. Further research is needed to determine how FGF23 excess impairs exercise capacity. The observation that cardiac function improves in CKD before changes in cardiac architecture are detectable suggests different time frames for the development of cardiac structural changes versus their resolution. Although addressing the underlying mechanisms of this structure-function divergence will require further research, this finding has immediate implications for future clinical research of the FGF23-cardiac axis in CKD. The results suggest that VO2 Max could serve as a primary outcome for phase 2 clinical trials to assess interventions that target the FGF23-cardiac axis more efficiently by enabling smaller sample sizes and shorter follow-up periods than would be required to detect changes in cardiac structure using imaging modalities. With this new approach and recent human genetic support for a causal effect of FGF23 on heart failure, particularly heart failure with preserved ejection fraction in patients who are genetically predisposed to develop CKD (13), the cardio-renal community should no longer exercise restraint over developing therapies to target the FGF23-cardiac axis.
Acknowledgments
The content of this article reflects the personal experience and views of the authors and should not be considered medical advice or recommendation. The content does not reflect the views or opinions of the American Society of Nephrology (ASN) or Kidney360. Responsibility for the information and views expressed herein lies entirely with the authors.
Footnotes
See related article, “FGF23 and Cardiovascular Structure and Function in Advanced Chronic Kidney Disease,” on pages 1529–1541.
Disclosure
M. Wolf reports consultancy for Akebia, Amgen, Bayer, Enyo, Jnana, Pharmacosmos, and Reata; ownership interest in Akebia, Unicycive, and Walden; research funding from CSL-Behring; honoraria from Akebia, Amgen, Bayer, Enyo, Jnana, Pharmacosmos, and Reata; and an advisory or leadership role for Akebia, Unicycive, and Walden. The remaining author has nothing to disclose.
Funding
None.
Author Contributions
Both authors were responsible for conceptualization and supervision, wrote the original draft of the manuscript, and reviewed and edited the manuscript.
References
- 1.Wolf M: Mineral (mal)adaptation to kidney disease—Young Investigator Award Address: American Society of Nephrology Kidney Week 2014. Clin J Am Soc Nephrol 10: 1875–1885, 2015. 10.2215/CJN.04430415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin KJ, González EA: Metabolic bone disease in chronic kidney disease. J Am Soc Nephrol 18: 875–885, 2007. 10.1681/ASN.2006070771 [DOI] [PubMed] [Google Scholar]
- 3.Shiizaki K, Tsubouchi A, Miura Y, Seo K, Kuchimaru T, Hayashi H, Iwazu Y, Miura M, Battulga B, Ohno N, Hara T, Kunishige R, Masutani M, Negishi K, Kario K, Kotani K, Yamada T, Nagata D, Komuro I, Itoh H, Kurosu H, Murata M, Kuro-O M: Calcium phosphate microcrystals in the renal tubular fluid accelerate chronic kidney disease progression. J Clin Invest 131: e145693, 2021. 10.1172/JCI145693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, Gutiérrez OM, Aguillon-Prada R, Lincoln J, Hare JM, Mundel P, Morales A, Scialla J, Fischer M, Soliman EZ, Chen J, Go AS, Rosas SE, Nessel L, Townsend RR, Feldman HI, St John Sutton M, Ojo A, Gadegbeku C, Di Marco GS, Reuter S, Kentrup D, Tiemann K, Brand M, Hill JA, Moe OW, Kuro-O M, Kusek JW, Keane MG, Wolf M: FGF23 induces left ventricular hypertrophy. J Clin Invest 121: 4393–4408, 2011. 10.1172/JCI46122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Isakova T, Xie H, Yang W, Xie D, Anderson AH, Scialla J, Wahl P, Gutiérrez OM, Steigerwalt S, He J, Schwartz S, Lo J, Ojo A, Sondheimer J, Hsu CY, Lash J, Leonard M, Kusek JW, Feldman HI, Wolf M; Chronic Renal Insufficiency Cohort (CRIC) Study Group : Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA 305: 2432–2439, 2011. 10.1001/jama.2011.826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehta R, Cai X, Lee J, Scialla JJ, Bansal N, Sondheimer JH, Chen J, Hamm LL, Ricardo AC, Navaneethan SD, Deo R, Rahman M, Feldman HI, Go AS, Isakova T, Wolf M; Chronic Renal Insufficiency Cohort (CRIC) Study Investigators : Association of fibroblast growth factor 23 with atrial fibrillation in chronic kidney disease, from the Chronic Renal Insufficiency Cohort Study. JAMA Cardiol 1: 548–556, 2016. 10.1001/jamacardio.2016.1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scialla JJ, Xie H, Rahman M, Anderson AH, Isakova T, Ojo A, Zhang X, Nessel L, Hamano T, Grunwald JE, Raj DS, Yang W, He J, Lash JP, Go AS, Kusek JW, Feldman H, Wolf M; Chronic Renal Insufficiency Cohort (CRIC) Study Investigators : Fibroblast growth factor-23 and cardiovascular events in CKD. J Am Soc Nephrol 25: 349–360, 2014. 10.1681/ASN.2013050465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Halim A, Burney HN, Li X, Li Y, Tomkins C, Siedlecki A, Lu T-S, Kalim S, Thadhani R, Moe S, Ting S, Zehnder D, Hiemstra T, Lim K: FGF23 and cardiovascular structure and function in advanced chronic kidney disease [published online ahead of print July 5, 2022]. Kidney360 10.34067/KID.0002192022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lim K, Ting SMS, Hamborg T, McGregor G, Oxborough D, Tomkins C, Xu D, Thadhani R, Lewis G, Bland R, Banerjee P, Fletcher S, Krishnan NS, Higgins R, Zehnder D, Hiemstra TF: Cardiovascular functional reserve before and after kidney transplant. JAMA Cardiol 5: 420–429, 2020. 10.1001/jamacardio.2019.5738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balady GJ, Arena R, Sietsema K, Myers J, Coke L, Fletcher GF, Forman D, Franklin B, Guazzi M, Gulati M, Keteyian SJ, Lavie CJ, Macko R, Mancini D, Milani RV; American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee of the Council on Clinical Cardiology; Council on Epidemiology and Prevention; Council on Peripheral Vascular Disease; Interdisciplinary Council on Quality of Care and Outcomes Research : Clinician’s guide to cardiopulmonary exercise testing in adults: A scientific statement from the American Heart Association. Circulation 122: 191–225, 2010. 10.1161/CIR.0b013e3181e52e69 [DOI] [PubMed] [Google Scholar]
- 11.Ghuman J, Cai X, Patel RB, Khan SS, Hecktman J, Redfield MM, Lewis G, Shah SJ, Wolf M, Isakova T, Mehta R: Fibroblast growth factor 23 and exercise capacity in heart failure with preserved ejection fraction. J Card Fail 27: 309–317, 2021. 10.1016/j.cardfail.2020.09.477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolf M, Weir MR, Kopyt N, Mannon RB, Von Visger J, Deng H, Yue S, Vincenti F: A prospective cohort study of mineral metabolism after kidney transplantation. Transplantation 100: 184–193, 2016. 10.1097/TP.0000000000000823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akwo E, Pike MM, Ertuglu LA, Vartanian N, Farber-Eger E, Lipworth L, Perwad F, Siew E, Hung A, Bansal N, de Boer I, Kestenbaum B, Cox NJ, Ikizler TA, Wells Q, Robinson-Cohen C: Association of genetically predicted fibroblast growth factor-23 with heart failure: A Mendelian randomization study. Clin J Am Soc Nephrol 17: 1183–1193, 2022. 10.2215/CJN.00960122 [DOI] [PMC free article] [PubMed] [Google Scholar]