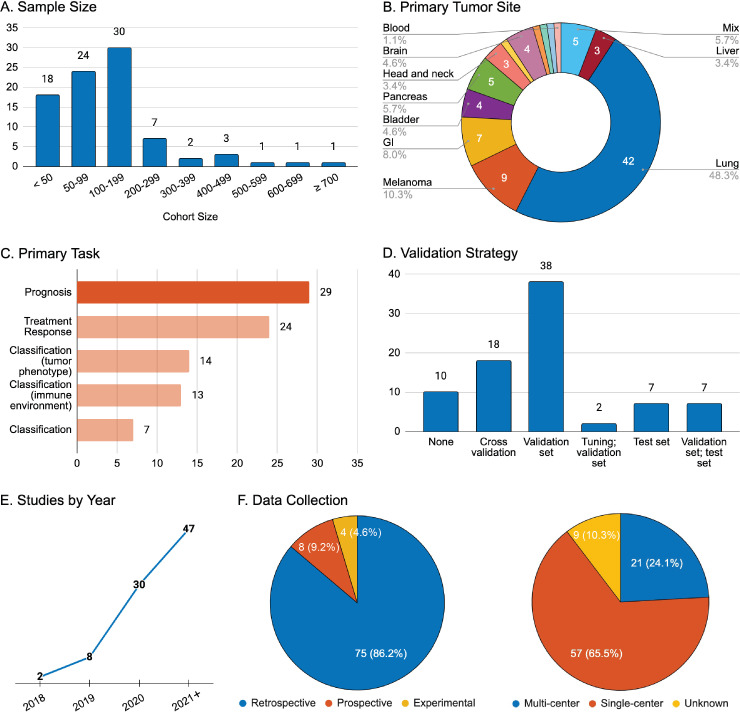
Figure 2.
General overview of study characteristics for reports involving radiomics and immunotherapy. (A) Aggregate number of patients included in the study for all purposes; (B) Primary tumor site of the disease investigated; (C) Stated task of the research: prognosis (overall survival, progression-free survival, durable clinical benefit), treatment response (defined by Response Evaluation Criteria in Solid Tumors (RECIST v1.1), tumor phenotype (programmed cell death-ligand 1 expression, microsatellite instability), immune environment (tumor immune cell infiltration), general classification (serious sequelae and adverse events from immunotherapy or adjuvant treatment); (D) Strategy for radiomics model performance validation; (E) Year of publication; (F) Data collection strategy and data source. GI, gastrointestinal.