Background
Wide-volume scanning with 320-row multidetector computed tomography coronary angiography (CTCA-WVS) enables the assessment of the aortic arch plaque (AAP) morphology and coronary arteries without requiring additional contrast volume. This study aimed to investigate the prevalence of AAPs and their association with coronary artery disease (CAD) and major adverse cardiovascular events (MACEs) in patients who underwent CTCA-WVS.
Methods
This study included 204 patients without known CAD (mean age, 65 years; 53% men) who underwent CTCA-WVS. We evaluated the presence of aortic plaques in the ascending aorta, aortic arch, and thoracic descending aorta using CTCA-WVS. Large aortic plaques were defined as plaques of at least 4 mm in thickness. A complex aortic plaque was defined as a plaque with ulceration or protrusion. MACEs were defined as composite events of cardiovascular (CV) death, nonfatal myocardial infarction, and ischemic stroke.
Results
AAPs and large/complex AAPs were identified in 51% (n = 105) and 18% (n = 36) of the study patients, respectively. The prevalence of AAPs with large/complex morphology increased with CAD severity (2.1% in no CAD, 12% in nonobstructive CAD, and 39% in obstructive CAD). The univariate Cox hazard model demonstrated that the predictors associated with MACEs were diabetes, obstructive CAD, and large/complex AAPs. Independent factors associated with large/complex AAPs were male sex [odds ratio (OR), 2.90; P = 0.025], stroke history (OR, 3.48; P = 0.026), obstructive CAD (OR, 3.35; P = 0.011), and thoracic aortic calcification (OR, 1.77; P = 0.005).
Conclusion
CTCA-WVS provides a comprehensive assessment of coronary atherosclerosis and thoracic aortic plaques in patients with CAD, which may improve the stratification of patients at risk for CV events.
Keywords: aortic arch plaque, coronary atherosclerosis, coronary computed tomography angiography, stroke
Introduction
Cardiovascular (CV) diseases, mainly comprising coronary artery disease (CAD) and stroke, are leading causes of mortality and morbidity and contribute significantly to the public health burden [1,2]. Previous echocardiography studies have mainly focused on the prevalence of aortic arch plaques (AAPs) as embolic sources among patients with stroke, atrial fibrillation, and valvular heart disease [3–5]. The presence of complex AAPs has been associated with ischemic stroke [odds ratio (OR), 17.1], whereas large, noncomplex plaques (≥4 mm) have been associated with a mildly increased risk of stroke (OR, 2.4) [6]. Based on these results, both large/complex and noncomplex AAPs may be used as prognostic markers for recurrent stroke. However, the prevalence and clinical significance of AAPs in patients with CAD remain largely unknown.
Computed tomography (CT) coronary angiography (CTCA) is a noninvasive imaging technology used to assess the coronary artery calcium score (CACS), CAD severity, and coronary plaque morphology [7,8], offering a first-line test for symptomatic patients with suspected CAD [9]. In addition to its diagnostic utility in obstructive CAD, CACS of at least 300 was found to serve as an imaging marker for predicting coronary death and myocardial infarction in individuals [10]. Previous clinical studies have demonstrated that thoracic aortic calcification (TAC) provides limited prognostic implications for CV events over the CACS [11–14]. Despite the reliable imaging quality used to assess coronary atherosclerosis, CTCA imaging has limited ability to assess the aortic arch, where TAC is frequently detected [15]. Recent advances in wide-range detector CT have eliminated redundant radiation exposure compared with helical scans, in which oversampling or overlapping of sequential axial images is acquired [16,17]. A wide-volume scan with a 320-row multidetector CT (320MDCT) coronary angiography (CTCA-WVS) covers a wide imaging range of approximately 16 cm in a volume scan [18,19] that visualizes a holistic image of the thoracic aorta, including the aortic arch. CTCA-WVS is also capable of simultaneously assessing both the aortic arch and the coronary arteries without additional contrast medium injection. This permits a comprehensive assessment of atherosclerotic disease burden in the thoracic aorta in parallel with the coronary arteries.
The present study aimed to investigate: (a) the prevalence and morphological features of aortic plaques and the determinants of large/complex AAPs in association with CACS and CAD severity and (b) the prognostic impact of large/complex AAPs in patients with suspected CAD who underwent CTCA-WVS.
Materials and methods
Study population
CTCA-WVS was performed in consecutive patients with or without known CAD at our institution to assess AAPs concurrent with CAD and to evaluate implanted coronary stents or coronary artery bypass grafts. The exclusion criteria for the CTCA-WVS examination conformed with the current standard clinical practice: (a) cardiogenic shock, (b) acute myocardial infarction, (c) advanced-stage chronic kidney disease (CKD) without maintenance hemodialysis, (d) a life expectancy of less than 1 year, and (e) pregnancy.
We retrospectively identified 231 consecutive patients who underwent CTCA-WVS between April 2017 and June 2018. To investigate the prevalence and morphological features of aortic plaques and the determinants of large/complex AAPs in association with CACS, patients with a history of coronary revascularization (n = 20) and/or open-heart surgery were excluded from the analysis (n = 7). The final study population comprised 204 patients without known CAD. Patients were classified into three categories according to coronary CT angiography findings: no CAD, nonobstructive CAD, or obstructive CAD. The study protocol was approved by the ethics committee of Fujiikai Kashibaseiki Hospital (approval number, 2021–B), and written informed consent for coronary angiography with 320MDCT was obtained from each patient. This study was conducted in accordance with the principles of the Declaration of Helsinki.
CTCA-WVS imaging protocol
All CTCA-WVS examinations were performed using 320MDCT (Aquilion ONE/NATURE Edition, Cannon Medical Systems, Inc., Tochigi, Japan) with two volume scans to evaluate the heart and aortic arch during a single breath-hold. The electrocardiogram-triggered prospective gating method was used for CTCA imaging. An oral beta-blocker was administered to patients with a heart rate of at least 60 bpm. The CACS and TAC were assessed at a fixed thickness of 3 mm using the Agatston scoring method. Following CACS imaging, the first volume scan was aimed at imaging the entire heart, whereas the second was aimed at imaging the aortic arch (Fig. 1). A bolus tracking method was used for image acquisition [20], and a nonionic contrast medium of 270 mg I/kg [ranging from 33 to 74 ml iopamidol (370 mg I/ml); Bracco, Milan, Italy] was administered with a power injector at a rate of 2.3–4.9 ml/s. After injection of the contrast medium, saline was injected through the same venous access site at the same injection rate. The region of interest was the ascending aorta at the bronchial bifurcation level. When the CT value exceeded 150 Hounsfield units, electrocardiogram-synchronized scans were performed within a single breath-hold. The scan parameters were as follows: detector collimation, 0.5 × 320 mm; gantry rotation time, 350 ms; tube voltage, 120 kV; and tube current, 130–600 mA. The scanning range included the aortic arch and entire heart.
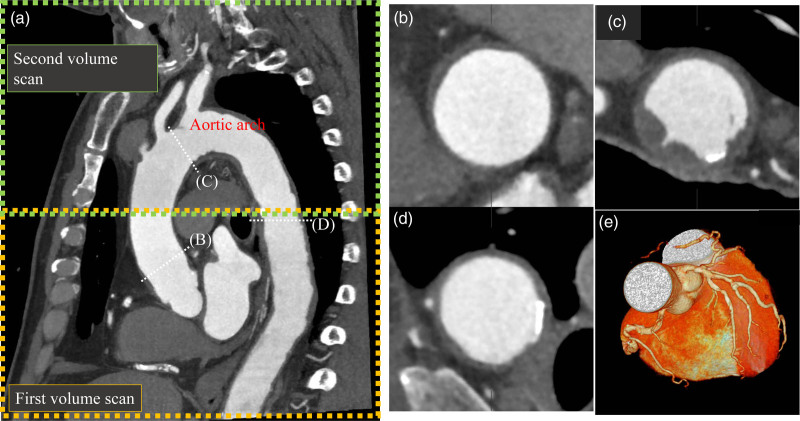
Fig. 1.
Computed tomography coronary angiography with wide-volume scanning for the aortic arch and coronary plaque imaging. (a) Oblique image of the thoracic aorta and heart. A combined image of the first and second volume scans (wide-volume scan) is automatically generated using 320-row multidetector computed tomography. (b) Aortic plaque in the ascending aorta. (c) Aortic arch plaque with protrusion. (d) Aortic plaque with calcification. (e) Volume rendering image of the coronary arteries.
Image interpretation and analysis
CTCA image analysis was performed using a dedicated software (VINCENT, Fujifilm Inc., Tokyo, Japan). Coronary artery diameter stenosis was reported based on a 16-segment American Heart Association model by two observers (K.O. and H.I.) [21]. Obstructive CAD was defined as the presence of stenosis of at least 50% of one or more major epicardial vessels and/or of at least 50% stenosis of the left main coronary segment. Nonobstructive CAD was defined as the presence of stenosis of less than 50% in one or more major epicardial vessels. Patients without atherosclerotic plaques were categorized as not having CAD. For the evaluation of CAD extent and severity, segment stenosis score (SSS) and involvement scores (SIS) were reported using the American Heart Association 16-segment model [21].
In this study, the thoracic aorta was categorized into three segments: ascending aorta, aortic arch, and descending aorta above the diaphragm. The ascending aorta was defined as the segment between the sinotubular junction and the origin of the innominate artery, the aortic arch segment was defined as the segment between the innominate artery and the aortic isthmus, and the descending aorta was defined as the segment between the aortic isthmus and the diaphragm (Fig. 1) [13]. As the images for scoring CACS did not include the aortic arch, we reported TAC as the sum of the ascending and descending aorta measurements, as previously described [11–14].
To report the mean CT attenuation value for each segment, the region of interest was placed in the cross-sectional images of each aortic segment (ascending aorta, aortic arch, and descending aorta). Aortic plaques have been reported in each segment of the aorta. Aortic plaques were defined as visible thickening of the aortic wall [13]. The thickness and arc of the aortic plaques were measured. A large aortic plaque was defined as a plaque of at least 4 mm in thickness, whereas a complex aortic plaque was defined as a plaque with ulceration or protrusion [3,22]. A representative image of an AAP is shown in Figs. 1 and 2.
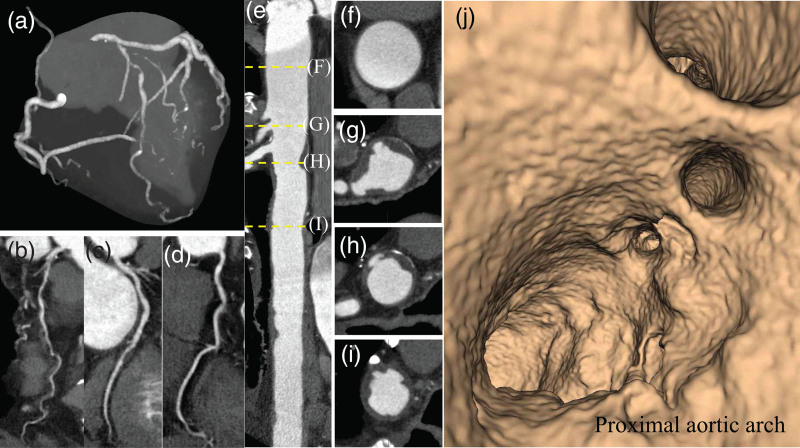
Fig. 2.
Computed tomography coronary angiography with wide-volume scanning images to visualize the thoracic aorta together with the coronary arteries in an obstructive CAD patient without stroke history. (a) Maximum intensity projection image of coronary arteries with multivessel obstructive CAD. Curved planner reconstruction images of LAD (b), LCX (c), and RCA (d). (e) Straight CPR image of contrast-enhanced CT angiography for the thoracic aorta. The ascending aorta (f) and ulcered AAPs ≥ 4 mm (g and h). (i) The descending aortic plaque with ulceration. (j) Fly through view of the aortic arch from proximal to distal aortic arch. Irregular luminal surface indicates complex AAPs partially corresponding to (g and h). AAPs, aortic arch plaques; CAD, coronary artery disease; CPR, curved planar reformation; LAD, left anterior descending coronary artery; LCX, left circumflex coronary artery; and RCA, right coronary artery.
Definition for major cardiovascular event
Clinical follow-up was performed through interviews with the patients during each hospital visit or via telephone and/or mail. A major adverse cardiovascular event (MACE) was defined as a composite event of CV death, nonfatal myocardial infarction, and ischemic stroke. Myocardial infarction was defined as persistent chest pain with cardiac enzyme elevation (elevation of cardiac troponin I above the reference level), regardless of ST-segment or non-ST-segment elevation. Ischemic stroke was defined as a sudden onset of neurological signs or symptoms fitting a focal or multifocal vascular territory within the brain, spinal cord, or retina that persisted for at least 24 h or until death, with neuroimaging evidence of a central nervous system infarction or absence of causation [23].
Statistical analysis
Statistical analyses were performed using SPSS version 24 (IBM Corp., Armonk, New York, USA). Categorical variables are reported as counts (percentage), and continuous variables are reported as mean (SD). Variables were compared using the Chi-square test for categorical variables, the t-test for normally distributed variables, and the Mann–Whitney U test for nonnormally distributed variables. Spearman’s rank correlation coefficient test was used to analyze the correlation between TAC and CACS, the number of aortic plaques, and the number of segments with large or complex aortic plaques. Univariate and multivariate logistic regression analysis was performed to examine whether obstructive CAD was independently associated with the presence of high-risk AAPs. The multivariate model was adjusted for known covariates of AAPs, including advanced age, male sex, diabetes, CKD, CACS, TAC, and stroke history [3,4,22,24]. Univariate Cox proportional hazard analysis was performed to determine predictors of MACEs. Using Kaplan–Meier curve analysis, a time-to-event analysis for patients with combinations of predictors was performed.
The inter- and intraobserver reproducibility of CTCA-WVS analyses was assessed on a randomly selected subset of 30 patients using kappa statistics for the presence of AAPs, complex AAPs, large AAPs, large/complex AAPs, and intraclass correlation coefficients (ICCs) for AAP thickness, if present. A P-value of <0.05 was considered to indicate statistical significance.
Results
Patient characteristics and computed tomography coronary angiography imaging
Patient characteristics are shown in Table 1. The mean patients had a mean age of 65 years (53% men). Patients with obstructive CAD were older (P < 0.001) and had higher serum C-reactive protein levels (P = 0.010) than those without CAD. Hypertension, atrial fibrillation, CKD, stroke history, and antiplatelet therapy were more frequently observed in patients with obstructive CAD than in those without CAD. As expected, the obstructive CAD group had greater CACS, SIS, and SSS than the other groups (all P < 0.001). There were no significant differences in the other clinical risk factors among the groups (Table 1).
Table 1.
Patient characteristics and computed tomography coronary angiography findings according to coronary artery disease presence
| Variables | Overall (n = 204) | No CAD (N = 46) | Nonobstructive CAD (N = 99) | Obstructive CAD (N = 59) | P-value for trend |
|---|---|---|---|---|---|
| Age, years | 64 (15) | 53 (14) | 67 (14) | 70 (11) | <0.001 |
| Sex, male | 108 (53%) | 23 (50%) | 52 (52%) | 33 (56%) | 0.82 |
| BMI, kg/mm2 | 24.1 (3.9) | 25.3 (5.5) | 23.9 (3.4) | 23.6 (3.2) | 0.07 |
| SBP, mmHg | 143 (22) | 138 (20) | 144 (22) | 144 (24) | 0.22 |
| DBP, mmHg | 80 (15) | 81 (13) | 80 (14) | 79 (17) | 0.92 |
| Heart rate, bpm | 74 (17) | 74 (15) | 72 (15) | 77 (20) | 0.15 |
| Hypertension | 143 (70%) | 25 (54%) | 72 (73%) | 46 (78%) | 0.02 |
| Diabetes | 40 (20%) | 5 (11%) | 18 (18%) | 17 (29%) | 0.06 |
| Dyslipidemia | 129 (63%) | 25 (54%) | 63 (64%) | 41 (69%) | 0.27 |
| Current smoker | 33 (16%) | 10 (22%) | 13 (13%) | 10 (17%) | 0.41 |
| Atrial fibrillation | 21 (10%) | 1 (2%) | 10 (10%) | 10 (17%) | 0.04 |
| Previous stroke history | 25 (12%) | 1 (2%) | 11 (11%) | 13 (22%) | 0.008 |
| Chronic kidney disease | 55 (27%) | 5 (11%) | 30 (30%) | 20 (34%) | 0.018 |
| Laboratory test | |||||
| HDL-cholesterol, mg/dl | 62 (18) | 61 (18) | 63 (18) | 61 (19) | 0.76 |
| LDL-cholesterol, mg/dl | 123 (33) | 128 (28) | 120 (31) | 118 (32) | 0.41 |
| HbA1c, % | 5.9 (0.9) | 5.8 (0.4) | 6.0 (1.1) | 6.2 (1.1) | 0.43 |
| CRP, g/dl | 0.39 (0.9) | 0.22 (0.46) | 0.24 (0.60) | 0.74 (1.5) | <0.001a |
| Medication | |||||
| Antiplatelet therapy, n (%) | 27(13) | 3 (7%) | 10 (10%) | 14 (24%) | 0.02 |
| RAS inhibitor, n (%) | 44(22) | 7 (15%) | 20 (20%) | 17 (29%) | 0.22 |
| Calcium-channel blocker, n (%) | 50(25) | 8 (53%) | 22 (22%) | 20 (34%) | 0.11 |
| Statin, n (%) | 47(23) | 7 (15%) | 24 (24%) | 16 (27%) | 0.33 |
| CCTA findings | |||||
| CACS | 204 (167) | 0 (0) | 120 (325) | 378 (524) | <0.001a |
| SIS | 2.02 (2.28) | 0 (0) | 1.6 (1.3) | 4.3 (2.6) | <0.001 |
| SSS | 4.47 (5.63) | 0 (0) | 2.9 (2.3) | 10.6 (6.6) | <0.001 |
| Location of vessels for obstructive CAD and extent of CAD | |||||
| LAD | 41 (20%) | - | - | 41 | - |
| LCX | 19 (9.3%) | - | - | 19 | - |
| RCA | 21 (8.8%) | - | - | 21 | - |
| CT attenuation value within ROI | |||||
| LMCA | 398 (72) | 378 (84) | 398 (65) | 413 (71) | 0.08 |
| Ascending aorta | 422 (75) | 401 (78) | 426 (74) | 430 (71) | 0.10 |
| Aortic arch | 336 (94) | 313 (94) | 339 (90) | 349 (98) | 0.14 |
| Descending aorta | 395 (172) | 392 (68) | 398 (76) | 391 (70) | 0.48 |
Variables are reported as number (%), mean (SD).
CACS, coronary artery calcium score; CRP, C-reactive protein; CTCA; computed tomography coronary angiography; HbA1c, hemoglobin A1c; LAD, left anterior descending coronary artery; LMCA, left main coronary artery; LCX, left circumflex coronary artery; MDCT, multidetector computed tomography; RCA, right coronary artery; ROI, region of interest; SIS, segment involvement score; SSS, stenosis severity score.
Log-transformation was used to assess statistical significance for CRP and CACS.
All CTCA-WVS images were available for interpretation and analyses. CT attenuation values for the left main coronary artery ostium and each aortic segment are presented in Table 1. Excellent inter- and intraobserver agreements were observed for the identification of AAPs (κ, 0.93 and 1.0), complex AAPs (κ, 0.92 and 0.92), large AAPs (κ, 0.85 and 0.93), and large/complex AAPs (κ, 0.86 and 0.93). Similar inter- and intraobserver ICCs were observed in the measurement of an AAP at the thickest part (ICC, 0.94 and 0.98, respectively).
Prevalence of aortic arch and thoracic aortic plaques
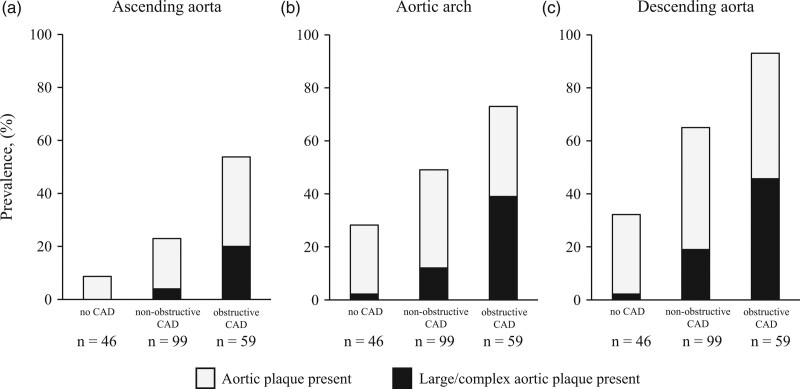
The CTCA-WVS findings according to CAD severity are summarized in Table 2. The prevalence of aortic plaques in each thoracic aortic segment is summarized in Fig. 3. Aortic arch and large/complex aortic plaques were observed in 51% (n = 105) and 18% (n = 36) of patients, respectively (Table 2 and Fig. 3). The prevalence of AAPs with large/complex morphology increased with the CAD severity (2.1% in no CAD, 12% in nonobstructive CAD, and 39% in obstructive CAD) (Table 2 and Fig. 3). Compared with patients without obstructive CAD, those with obstructive CAD exhibited a greater number of segments with aortic plaques and large/complex aortic plaques (Table 2). Patients with obstructive CAD had greater TACs than those without (P < 0.001; Table 2). Compared with patients without obstructive CAD, those with obstructive CAD had significantly greater TACs (P < 0.001; Table 2). Spearman’s rank correlation coefficient test demonstrated a significant correlation between TAC (logTAC+1) and CACS (logCACS+1; ρ = 0.601; P < 0.001), number of aortic plaques (ρ = 0.613; P < 0.001), and large/complex aortic plaques (ρ = 0.452; P < 0.001).
Table 2.
Computed tomography coronary angiography findings aortic plaque according to coronary artery calcium score category
| Variables | Overall (n = 204) | No CAD (N = 46) | Nonobstructive CAD (N = 99) | Obstructive CAD (N = 59) | P-value for trend |
|---|---|---|---|---|---|
| Ascending aorta segment | |||||
| Ascending aortic plaque | 59 (29%) | 4 (8.6%) | 23 (23%) | 32 (54%) | <0.001 |
| Complex plaque (ulcered or protruded plaque) | 12 (5.8%) | 0 (0%) | 2 (2.0%) | 10 (17%) | <0.001 |
| Large plaque ≥4 mm in thickness | 5 (2.5%) | 0 (0%) | 2 (2.0%) | 3 (5.1%) | 0.23 |
| Large or complex plaque | 16 (7.8%) | 0 (0%) | 4 (4.0%) | 12 (20%) | <0.001 |
| Aortic arch segment | |||||
| Aortic arch plaque (AAP) | 105 (51%) | 13 (28%) | 49 (49%) | 43 (73%) | <0.001 |
| Complex plaque (ulcered or protruded plaque) | 20 (9.8%) | 0 (0%) | 7 (7.1%) | 23 (39%) | <0.001 |
| Large plaque ≥4 mm in thickness | 25 (12%) | 1 (2.1%) | 7 (7.1%) | 17 (29%) | <0.001 |
| Large or complex plaque | 36 (18%) | 1 (2.1%) | 12 (12%) | 23 (39%) | <0.001 |
| Descending aorta segment | |||||
| Descending aortic plaque | 134 (66%) | 15 (33%) | 64 (65%) | 55 (93%) | <0.001 |
| Complex plaque (ulcered or protruded plaque) | 44 (22%) | 1 (2.2%) | 17 (17%) | 26 (44%) | <0.001 |
| Large plaque ≥4 mm in thickness | 21 (10%) | 1 (2.2%) | 7 (7.1%) | 13 (22%) | 0.001 |
| Large or complex plaque | 47 (23%) | 1 (2.2%) | 19 (19%) | 27 (46%) | <0.001 |
| Number of segments with aortic plaque | |||||
| Aortic plaque present | |||||
| 0 segment | 58 (28%) | 29 (63%) | 25 (25%) | 4 (6.7%) | <0.001 |
| 1 segment | 44 (22%) | 6 (13%) | 31 (31%) | 7 (12%) | 0.004 |
| 2 segments | 52 (25%) | 7 (15%) | 24 (24%) | 21 (36%) | 0.055 |
| 3 segments | 50 (25%) | 4 (8.6%) | 19 (19%) | 27 (46%) | <0.001 |
| Number of segment with large/complex plaque | |||||
| 0 segment | 143 (70%) | 44 (96%) | 75 (75%) | 24 (41%) | <0.001 |
| 1 segment | 30 (15%) | 2 (4.3%) | 14 (14%) | 14 (24%) | 0.020 |
| 2 segments | 24 (12%) | 0 (0%) | 9 (9.0%) | 15 (25%) | <0.001 |
| 3 segments | 7 (3.4%) | 0 (0%) | 1 (1.0%) | 6 (10%) | 0.003 |
| TAC | 1193 (2791) | 283 (1356) | 983 (2209) | 2225 (2791) | <0.001a |
Variables are reported as number (%) or mean (SD).
CACS, coronary artery calcium score; CTCA, computed tomography coronary angiography.
Log-transformation was used to assess statistical significance for TAC.
Fig. 3.
Prevalence of aortic plaques with or without large/complex morphology according to CAD severity. The prevalence of aortic plaques and large/complex aortic plaques in the ascending aorta (a), aortic arch (b), and descending aorta (c). Prevalence of aortic plaques with or without large/complex morphology increased along with the presence and severity of CAD in all the segments (all P < 0.001). CAD, coronary artery disease; CACS, coronary artery calcium score.
Predictors of high-risk featured aortic arch plaques
Table 3 shows the associations between clinical characteristics and CTCA findings with large/complex AAPs. In the univariate analysis, predictors associated with the presence of large/complex AAPs were diabetes [OR, 2.95; 95% confidence interval (CI), 1.333–6.536; P < 0.001], stroke history (OR, 4.84; 95% CI, 1.976–11.853; P = 0.001), obstructive CAD (OR, 6.48; 95% CI, 1.234–9.622; P < 0.001), multivessel disease (OR, 3.44; 95% CI, 1.234–9.622; P = 0.018), CACS (OR, 2.22; 95% CI, 1.558–3.184; P < 0.001), and TAC (OR, 2.02; 95% CI, 1.500–2.727; P < 0.001). In the multivariate analysis adjusted for advanced age, diabetes, CACS, and CKD, male sex (OR, 2.90; 95% CI, 1.145–7.363; P = 0.025), stroke history (OR, 3.48; 95% CI, 1.159–10.464; P = 0.026), obstructive CAD (OR, 3.35; 95% CI, 1.325–8.484; P = 0.011), and TAC (OR, 1.77; 95% CI, 1.191–2.640; P = 0.005) were independently associated with the presence of large/complex AAPs detected by CTCA-WVS.
Table 3.
Univariate and multivariate logistic regression analysis to predict large/complex aortic arch plaques on wide-volume scanning with 320-row multidetector computed tomography coronary angiography
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Age ≥ 75 years old | 2.07 (0.983–4.386) | 0.055 | 0.79 (0.303–2.072) | 0.635 |
| Male sex | 1.72 (0.821–3.637) | 0.150 | 2.90 (1.145–7.363) | 0.025 |
| Hypertension | 1.61 (0.689–3.776) | 0.271 | ||
| Diabetes | 2.95 (1.333–6.536) | <0.001 | 1.34 (0.497–3.650) | 0.558 |
| CKD | 1.69 (0.789–3.642) | 0.176 | 1.09 (0.426–2.780) | 0.860 |
| Previous stroke history | 4.84 (1.976–11.853) | 0.001 | 3.48 (1.159–10.464) | 0.026 |
| Obstructive CAD | 6.48 (3.993–14.060) | <0.001 | 3.35 (1.325–8.484) | 0.011 |
| Log (CACS+1) | 2.22 (1.558–3.184) | <0.001 | 1.15 (0.713–1.875) | 0.557 |
| Log (TAC+1) | 2.02 (1.500–2.727) | <0.001 | 1.77 (1.191–2.640) | 0.005 |
CACS and TAC was analyzed by log transformation.
CAD, coronary artery disease; CTCA, computed tomography coronary angiography; CKD, chronic kidney disease; CACS, coronary artery calcium score; OR, odds ratio; TAC, thoracic artery calcium; WVS, wide-volume scanning.
Predictors of major cardiovascular events
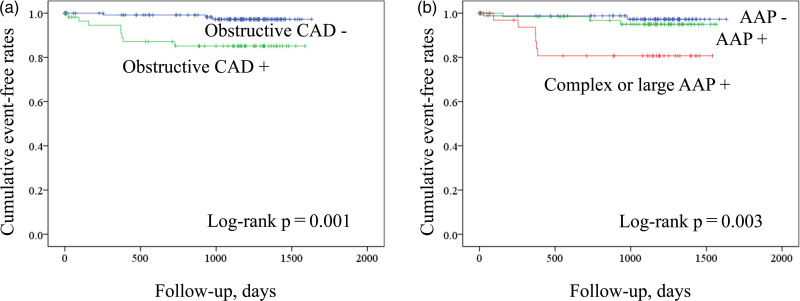
During a mean follow-up period of 2.7 ± 1.3 years, MACEs were observed in 11/204 patients (5.4%; two CV deaths, four nonfatal myocardial infarctions, and five ischemic strokes). Table 3 shows the univariate analysis using the Cox proportional hazards model to predict MACEs during the follow-up period. Univariate Cox hazard proportional model revealed that diabetes [hazard ratio (HR), 3.60; P = 0.034], obstructive CAD (HR, 6.58; P = 0.005), CACS (HR, 1.92; P = 0.024), TAC (HR, 1.88; P = 0.011), large/complex AAPs (HR, 6.11; P = 0.003), number of aortic plaque segments (HR, 2.00; P = 0.034), and number of segments with large/complex aortic plaques (HR, 2.64; P < 0.001) were associated with MACEs (Table 4). Figure 4 shows the Kaplan–Meier curve analysis for MACE prediction according to the presence or absence of obstructive CAD (Fig. 4a) and AAPs with and without complex or large plaque morphology (Fig. 4b).
Table 4.
Univariate Cox hazard proportional analysis for prediction of major adverse cardiovascular event
| Variables | Univariate analysis | |
|---|---|---|
| Hazard ratio (95% CI) | P-value | |
| Age ≥ 75 years | 3.23 (0.987–10.607) | 0.053 |
| Sex, male | 1.56 (0.457–5.337) | 0.477 |
| Diabetes | 3.60 (1.10–11.832) | 0.034 |
| CKD | 2.11 (0.646–6.939) | 0.215 |
| Obstructive CAD | 6.58 (1.742–24.765) | 0.005 |
| CACS (LogCACS+1) | 1.92 (1.091–3.411) | 0.024 |
| TAC (LogTAC+1) | 1.88 (1.154–3.092) | 0.011 |
| Large/complex AAP | 6.11 (1.86–20.070) | 0.003 |
| Number of segments with aortic plaque | 2.00 (1.055–3.825) | 0.034 |
| Number of segments with large/complex aortic plaque | 2.64 (1.577–4.570) | <0.001 |
AAP, aortic arch plaque; CAD, coronary artery disease; CACS, coronary artery calcium score; CKD, chronic kidney disease; MACE, major cardiovascular events; TAC, thoracic aortic calcification.
Fig. 4.
Kaplan–Meier curve analysis according to the presence or absence of predictors. (a) Patients were stratified according to the presence of obstructive coronary artery disease. (b) Patients were stratified by the presence of aortic arch plaques with or without large/complex morphology.
Discussion
This CTCA-WVS study evaluated the prevalence, extent, and prognostic significance of aortic plaques in patients with suspected CAD. The number of aortic plaques with and without a large/complex morphology increased with the severity of CAD. Obstructive CAD, previous stroke history, and TAC were associated with the presence of large/complex AAPs, independent of clinical risk factors. The extent of aortic plaques with or without a large/complex morphology was associated with an increased risk for MACEs.
Aortic arch plaques as a cause of embolic stroke and cardiovascular event
In addition to aortic calcification, transesophageal echocardiography (TEE) studies have demonstrated significant associations between AAP morphology and recurrent stroke, cognitive dysfunction, and brain aging [3,22,24]. These observations have deepened our understanding of AAPs as sources of embolic stroke and markers of CV events. CTCA is a noninvasive imaging modality that enables CAD severity assessment but provides limited data for atherosclerotic alterations in the aortic arch. With the dissemination of clinical applications of CTCA [11–14], TAC has provided prognostic implications for CV events beyond the embolic stroke source. However, the prevalence of aortic plaques with or without large/complex morphology in patients with suspected CAD remains unknown. In the present CTCA-WVS study, we observed an increased prevalence of large/complex AAP morphology, along with an increase in CAD severity. In line with our observations, Fujita et al. [3] investigated patients with aortic stenosis who underwent TEE and reported that patients with concomitant CAD had a higher prevalence of large or complex morphological AAPs than did those without CAD. The close association between the presence of AAPs and CV diseases may indicate that AAPs can serve as markers of systemic atherosclerotic disease burden beyond the role of embolic stroke.
Clinical implication of aortic plaque identification in coronary artery disease
In the present study, we observed an independent association between obstructive CAD and the presence of large/complex AAP morphology even after adjusting for clinical risk factors and TAC. In a recent study using nonobstructive angioscopy, Komatsu et al. [25] evaluated the prevalence of spontaneous aortic plaque rupture and unique pathological observations among patients who underwent coronary angiography (n = 262). In that study, spontaneously ruptured aortic plaques were observed in 80.9% of patients, of which 54% were detected in the thoracic aorta above the diaphragm. These observations highlight the importance of the comprehensive assessment and management of aortic arch atherosclerosis in patients with CAD to evaluate the individual atherosclerotic disease burden.
CTCA is a first-line noninvasive method for diagnosing CAD in patients [9] and has been shown to improve the outcomes of these patients by providing information on the presence of CAD [26]. In contrast, large/complex AAPs can serve as markers of mortality and ischemic stroke [6]. We observed that the number of segments with aortic plaques with and without large/complex morphology was associated with MACEs. This result may improve the risk stratification of CV events in patients without an overt stroke. Further studies are needed to investigate whether the combination of obstructive CAD and large/complex AAPs can serve as an independent outcome predictor in patients with CAD undergoing CTCA-WVS.
In the present study, we observed that one-third of the patients without CAD and half of those with nonobstructive CAD exhibited AAP, whereas most patients did not receive statin therapy (23% of the study population). Current guidelines recommend antiplatelet and statin therapies for patients with ischemic stroke or transient ischemic attack with evidence of AAP [1]. In patients with CAD and evidence of coronary atherosclerosis, statin therapy is recommended for acute coronary syndrome prevention, and antiatherosclerotic therapy is recommended based on long-term CV risk estimation [27]. Using CTCA-WVS enables the comprehensive assessment of CAD and AAPs, which may offer unique opportunities for the management of patients with CAD and AAP. Further studies are warranted to determine whether early identification of AAP using CTCA-WVS provides prognostic and therapeutic implications in patients with suspected CAD.
Study limitations
This study had several limitations. First, performing two wide-volume scans increases the radiation dose when compared with single-volume scanning, although the wide-volume scan has been shown to use a reduced radiation dose compared with helical CT scans [16,17]. In addition to not requiring further contrast medium injections during the CTCA examination, this method eliminates redundant radiation exposure compared with the helical scan, which requires oversampling or overlapping of sequential axial images [16,17]. Second, 320MDCT coronary angiography has a limited ability to assess aortic mobile plaques as a complex morphology; however, the majority of mobile plaques are concomitant with complex plaque features [4]. Third, this study included consecutive patients without known CAD, which is a strength of this study because it does not entail any selection bias for the CTCA-WVS examination. However, patients with severe CKD were excluded from the CTCA examination in accordance with the current clinical practice, which may have caused a selection bias. Moreover, this might have affected the AAP predictors, as reported in a previous TEE study [4]. Finally, a therapeutic strategy for detecting large/complex AAPs using imaging modalities has not yet been developed. Using the CTCA-WVS may help elucidate the response of AAPs to comprehensive antiatherosclerotic therapies, including lifestyle modification, antiplatelet therapy, and intensive statin therapy. Further studies are needed to investigate whether the combination of obstructive CAD and large/complex AAPs can serve as an independent outcome predictor in patients with CAD undergoing CTCA-WVS.
Conclusion
CTCA-WVS offers a unique opportunity to assess AAPs and coronary atherosclerosis. Further studies are warranted to investigate the underlying mechanisms of aortic arch atherosclerosis that link CAD and cerebrovascular diseases.
Acknowledgements
The authors would like to thank the Department of Radiology Laboratory of Fujiikai Kashibaseiki Hospital for their contribution to CTCA imaging data acquisition.
This work was partially supported by a research grant from the Fukuda Foundation for Medical Technology.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI, Ezekowitz MD, et al. ; American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Peripheral Vascular Disease. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014; 45:2160–2236. [DOI] [PubMed] [Google Scholar]
- 2.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. ; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation. 2019; 139:e56–e528. [DOI] [PubMed] [Google Scholar]
- 3.Fujita S, Sugioka K, Matsumura Y, Ito A, Hozumi T, Hasegawa T, et al. Impact of concomitant coronary artery disease on atherosclerotic plaques in the aortic arch in patients with severe aortic stenosis. Clin Cardiol. 2013; 36:352–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matsumura Y, Sugioka K, Fujita S, Ito A, Iwata S, Yoshiyama M. Association between chronic kidney disease and thoracic aortic atherosclerosis detected using transesophageal echocardiography. Atherosclerosis. 2014; 237:301–306. [DOI] [PubMed] [Google Scholar]
- 5.Suzuki M, Furuya K, Ozawa M, Miura K, Ozawa T, Matsuzono K, et al. Complex aortic arch atherosclerosis in acute ischemic stroke patients with non-valvular atrial fibrillation. J Atheroscler Thromb. 2021; 28:776–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Tullio MR, Sacco RL, Savoia MT, Sciacca RR, Homma S. Aortic atheroma morphology and the risk of ischemic stroke in a multiethnic population. Am Heart J. 2000; 139:329–336. [DOI] [PubMed] [Google Scholar]
- 7.Otsuka K, Fukuda S, Tanaka A, Nakanishi K, Taguchi H, Yoshikawa J, et al. Napkin-ring sign on coronary CT angiography for the prediction of acute coronary syndrome. JACC Cardiovasc Imaging. 2013; 6:448–457. [DOI] [PubMed] [Google Scholar]
- 8.Abdelrahman KM, Chen MY, Dey AK, Virmani R, Finn AV, Khamis RY, et al. Coronary computed tomography angiography from clinical uses to emerging technologies: JACC state-of-the-art review. J Am Coll Cardiol. 2020; 76:1226–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolk MJ, Bailey SR, Doherty JU, Douglas PS, Hendel RC, Kramer CM, et al. ; American College of Cardiology Foundation Appropriate Use Criteria Task Force. ACCF/AHA/ASE/ASNC/HFSA/HRS/SCAI/SCCT/SCMR/STS 2013 multimodality appropriate use criteria for the detection and risk assessment of stable ischemic heart disease: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2014; 63:380–406. [DOI] [PubMed] [Google Scholar]
- 10.Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008; 358:1336–1345. [DOI] [PubMed] [Google Scholar]
- 11.Budoff MJ, Nasir K, Katz R, Takasu J, Carr JJ, Wong ND, et al. Thoracic aortic calcification and coronary heart disease events: the multi-ethnic study of atherosclerosis (MESA). Atherosclerosis. 2011; 215:196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoffmann U, Massaro JM, D’Agostino RB, Sr, Kathiresan S, Fox CS, O’Donnell CJ. Cardiovascular event prediction and risk reclassification by coronary, aortic, and valvular calcification in the Framingham heart study. J Am Heart Assoc. 2016; 5:e003144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurra V, Lieber ML, Sola S, Kalahasti V, Hammer D, Gimple S, et al. Extent of thoracic aortic atheroma burden and long-term mortality after cardiothoracic surgery: a computed tomography study. JACC Cardiovasc Imaging. 2010; 3:1020–1029. [DOI] [PubMed] [Google Scholar]
- 14.Kälsch H, Mahabadi AA, Moebus S, Reinsch N, Budde T, Hoffmann B, et al. Association of progressive thoracic aortic calcification with future cardiovascular events and all-cause mortality: ability to improve risk prediction? Results of the Heinz Nixdorf Recall (HNR) study. Eur Heart J Cardiovasc Imaging. 2019; 20:709–717. [DOI] [PubMed] [Google Scholar]
- 15.Craiem D, Chironi G, Casciaro ME, Graf S, Simon A. Calcifications of the thoracic aorta on extended non-contrast-enhanced cardiac CT. PLoS One. 2014; 9:e109584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsiao EM, Rybicki FJ, Steigner M. CT coronary angiography: 256-slice and 320-detector row scanners. Curr Cardiol Rep. 2010; 12:68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang EJ. Clinical Applications of wide-detector CT scanners for cardiothoracic imaging: an update. Korean J Radiol. 2019; 20:1583–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chimura M, Ohtani T, Yokoi K, Shiraki T, Katsimichas T, Kitao T, et al. Diagnostic performance of coronary angiography utilizing intraprocedural 320-row computed tomography with minimal contrast medium. Heart Vessels. 2020; 35:1341–1348. [DOI] [PubMed] [Google Scholar]
- 19.Koyanagi H, Tsutsumi Y, Tokuda Y, Tanaka A, Endo M, Furukawa Y, Abe S. Computed tomography imaging using split-bolus contrast injection with volume scan of aortic root and heart for preoperative evaluation of transcatheter aortic valve implantation. Heart Vessels. 2022; 37:132–141. [DOI] [PubMed] [Google Scholar]
- 20.Kai N, Oda S, Utsunomiya D, Nakaura T, Funama Y, Kidoh M, et al. Dual-region-of-interest bolus-tracking technique for coronary computed tomographic angiography on a 320-row scanner: reduction in the interpatient variability of arterial contrast enhancement. Br J Radiol. 2018; 91:20170541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi R, Shi K, Yang ZG, Guo YK, Diao KY, Gao Y, et al. Serial coronary computed tomography angiography-verified coronary plaque progression: comparison of stented patients with or without diabetes. Cardiovasc Diabetol. 2019; 18:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Di Tullio MR, Russo C, Jin Z, Sacco RL, Mohr JP, Homma S; Patent Foramen Ovale in Cryptogenic Stroke Study Investigators. Aortic arch plaques and risk of recurrent stroke and death. Circulation. 2009; 119:2376–2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lansky AJ, Messé SR, Brickman AM, Dwyer M, van der Worp HB, Lazar RM, et al. Proposed standardized neurological endpoints for cardiovascular clinical trials: an academic research consortium initiative. J Am Coll Cardiol. 2017; 69:679–691. [DOI] [PubMed] [Google Scholar]
- 24.Gupta A, Giambrone AE, Gialdini G, Finn C, Delgado D, Gutierrez J, et al. Silent brain infarction and risk of future stroke: a systematic review and meta-analysis. Stroke. 2016; 47:719–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Komatsu S, Yutani C, Ohara T, Takahashi S, Takewa M, Hirayama A, Kodama K. Angioscopic evaluation of spontaneously ruptured aortic plaques. J Am Coll Cardiol. 2018; 71:2893–2902. [DOI] [PubMed] [Google Scholar]
- 26.Newby DE, Adamson PD, Berry C, Boon NA, Dweck MR, Flather M, et al. ; SCOT-HEART Investigators. Coronary CT angiography and 5-year risk of myocardial infarction. N Engl J Med. 2018; 379:924–933. [DOI] [PubMed] [Google Scholar]
- 27.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019; 140:e596–e646. [DOI] [PMC free article] [PubMed] [Google Scholar]