Abstract
Background and Aims
Vaccine response is a concern in hemodialysis patients. Given that hemodialysis patients were not included in clinical trials, we aimed to synthesize the available evidence on the immunogenicity of coronavirus disease 2019 (COVID‐19) mRNA vaccines in hemodialysis patients.
Methods
We searched Scopus, PubMed, Sciencedirect, and finally google scholar databases for studies on COVID‐19 mRNA‐vaccines immunogenicity in hemodialysis patients up to December 1, 2021. Eligible articles measured antibodies against severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) spike or Receptor‐Binding Domain Antibody (S/RBD) postimmunization with COVID‐19 mRNA vaccines. The immunogenicity of the vaccine was evaluated using seroconversion rates measured between 21 and 30 days after the first immunization and between 14 and 36 days post the second dose. We included studies including participants without a history of COVID‐19 before vaccination. Healthy controls or health‐care workers served as the control groups. After selecting eligible articles, the data were finally extracted from included articles. We used a random effects model to estimate the pooled seroconversion rate after COVID‐19 mRNA vaccine administration. We assessed the heterogeneity between studies with the I 2 statistical index.
Result
We selected 39 eligible citations comprising 806 cases and 336 controls for the first dose and 6314 cases and 927 controls for the second dose for statistical analysis. After the first dose of mRNA vaccines, the seroconversion rate was 36% (95% confidence interval [CI]: 0.24–0.47) and 68% (95% CI: 0.45–0.91) in hemodialysis patients and the control group, respectively. While seroconversion rate after the second dose of mRNA vaccines was 86% (95% CI: 0.81–0.91) and 100% (95% CI: 1.00–1.00) in hemodialysis patients and the control group, respectively.
Conclusion
Although the immune response of hemodialysis patients to the second dose of the SARS‐CoV‐2 mRNA vaccine is very promising, the seroconversion rate of dialysis patients is lower than healthy controls. Periodically assessment of antibody levels of hemodialysis patients at short intervals is recommended.
Keywords: chronic kidney disease, COVID‐19 mRNA vaccines, hemodialysis, SARS‐CoV‐2, the seroconversion rate, vaccine immunogenicity
1. INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) was detected in late 2019 in Wuhan, China. 1 , 2 Coronavirus disease 2019 (COVID‐19) is one of the most critical health problems in the world right now. 3 , 4 , 5 Several vaccines have been licensed for emergency use. 6 , 7 Vaccination remains an essential part of preventive care due to the high infection rate in hemodialysis patients. As kidney function decreases, the antibody response to the vaccine is impaired. Different methods have been used to improve response to influenza A and hepatitis B vaccines, such as higher vaccine doses and more frequent booster vaccinations. 8 Vaccine efficacy in clinical trials has been determined for the general population. However, its efficacy has not been evaluated for vulnerable populations such as hemodialysis patients.
Hemodialysis patients are at high risk in the COVID‐19 pandemic due to increased average age, immunosuppression, renal failure, and frequent visits to dialysis centers. The mortality rate of COVID‐19 in these patients is much higher than in the general population, and up to 32% has been reported. 8 , 9 Including patients with kidney disease in the COVID‐19 vaccine, clinical trials are low. In most trials, people with “severe” or “chronic” kidney disease and people using/undergoing immunosuppression have been excluded. There is not much information about the effectiveness of COVID‐19 vaccines in hemodialysis patients. On the other hand, the effectiveness of previous vaccines such as hepatitis B and influenza in hemodialysis patients has been less than in the general population. The seroconversion rate after influenza virus vaccination is about 33%–80% in hemodialysis patients. 10
The combination of this evidence has raised concerns about the vaccine's efficacy in this group and raised questions: The efficacy of COVID‐19 vaccines and the rate of postvaccination seroresponse in hemodialysis patients has not been fully determined; the duration of immune protection after vaccination is unknown. Seroconversion is related to immune protection from many pathogens, and there is increasing evidence that the same is true for SARS‐CoV‐2. Some studies have found a strong correlation between spike1 antibody titer and neutralization ability, innate immunity and the recruitment of T‐cell‐specific SARS‐CoV‐2 responses. 11
Our objective was to synthesize the available evidence on the immunogenicity of COVID‐19 mRNA vaccines in hemodialysis patients compared with healthy controls.
2. METHODS
2.1. Systematic literature search
We conducted a systematic bibliographic search in the PubMed, Scopus, and Web of Science databases up to December 1, 2021, using the following keywords: (Severe acute respiratory syndrome coronavirus 2 or SARS‐CoV‐2) AND (Coronavirus Disease 2019 or COVID‐19) AND (vaccine efficacy) AND (hemodialysis or dialysis or kidney failure or chronic kidney disease) AND (seroresponse or antibody response or humoral response or serotiter or immunogenicity, effectiveness, or efficacy).
2.2. Eligibility criteria and study selection
Cohort and case–control studies published as of August 1, 2021, were searched. Other publications such as case reports, comments, conference abstracts, and review articles were excluded. We included all original studies on the efficacy or immunogenicity of COVID‐19 mRNA vaccines on hemodialysis patients. Studies written in languages other than English were excluded. Studies with participants without previous or active COVID‐19 infection met the inclusion criteria. In addition, the included studies measured immunoglobulin G (IgG) antibodies against SARS‐CoV‐ 2 S‐protein or RBD fragment. Vaccine immunogenicity was evaluated using seroconversion rates measured between 21 and 30 days after the first immunization and between 14 and 36 days post the second dose of COVID‐19 mRNA vaccines. Healthy controls or health‐care workers served as the control groups. The title and abstract of the articles were read. In the next step, the full text of the articles was evaluated for eligibility. After selecting the relevant studies, the reference list of each article was searched manually. Finally, all eligible articles were included in the study.
2.3. Data extraction
Two researchers independently recorded the following data: first author, year of study, country, type of study, number of cases, number of positive cases, number of the control group, number of positive controls, vaccine type, antibody type, timing post first/second dose (days). Discrepancies among the researchers were resolved through discussions or additional consultations with the third author.
2.4. Statistical analysis
The heterogeneity of studies was assessed using Cochran's Q test and the I 2 index. Due to the high heterogeneity, the random effect model was selected for meta‐analysis. Meta‐regression analysis was used to investigate the relationship between vaccine type and seroconversion rate. We used STATA version 11(STATA Corporation) for the analysis. All levels of significance tests were two‐sided and p‐values less than 0.05 was considered significant.
3. RESULTS
In the first research phase, a total of 2407 relevant articles were identified. After removing 2120 duplicate articles, 287 publications were reviewed for the title and abstract. Of these, 203 were removed due to irrelevance. The full text of another 84 articles was reviewed for eligibility criteria, and 45 articles were excluded due to insufficient data and no measurements of IgG antibody responses against S and RBD. Finally, 39 articles, 8 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 including 806 cases and 336 controls for the first dose and 6314 cases and 927 controls for the second dose, for evaluating immunogenicity were included in the meta‐analysis. The flowchart of the article selection process is shown in Figure 1. Details of all included studies are provided in Tables 1 and 2.
Figure 1.
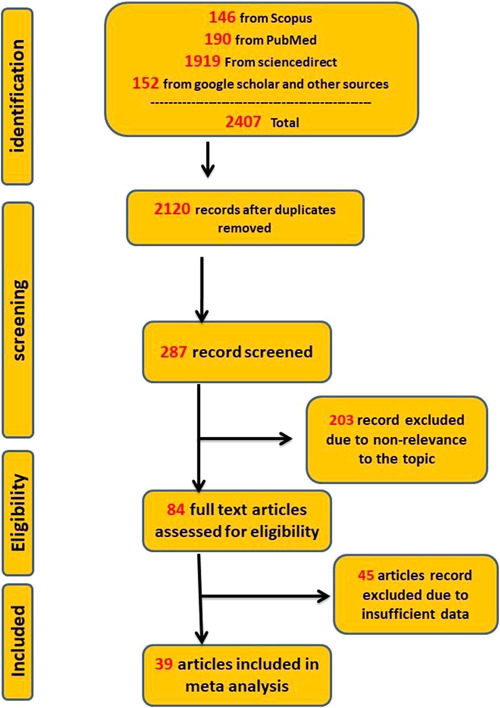
Flowchart of article identification and selection in the meta‐analysis
Table 1.
Antibody response data in dialysis patients after the first dose of mRNA vaccines
| (8, 12‐15) | Year | Country | Type of study | Number of cases | Number of positive cases (%) | Number of the control group | Number of positive controls (%) | Vaccine type | Antibody type | Timing post first dose (days) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Goupil et al. 12 | 2021 | Canada | Case–Control | 131 | 56 (43%) | 20 | 19 (95%) | BNT162b2 | Anti‐RBD IgG | 28 |
| 2 | Torregiani et al. 13 | 2021 | France | Cohort | 95 | 35 (36.84) | None | None | BNT162b2 | Anti‐S IgG | 21 |
| 3 | Speer et al. 8 | 2021 | Germany | Case–Control | 22 | 4 (18%) | 46 | 43 (93%) | BNT162b2 | Anti‐S1 IgG | 17–22 |
| 4 | Yau et al. 14 | 2021 | Canada | Case–Control | 66 | 53 (80%) | 35 | None | BNT162b2 | Anti‐S IgG | 28 |
| 5 | Attias et al. 15 | 2021 | France | Cohort | 56 | 10 (18%) | None | None | BNT162b2 | Anti‐S1 IgG | 28 |
| 6 | Longline et al. 16 | 2021 | France | Cohort | 80 | 17 (21.5%) | None | None | BNT162b2 | Anti‐RBD TOTAL | 30 |
| 7 | Weigert et al. 17 | 2021 | Portugal | Case–Control | 143 | 42 (29.4%) | 143 | 71 (49/7%) | BNT162b2 | Anti S‐IgG | 21 |
| 8 | Broseta et al. 18 | 2021 | Spain | Cohort | 78 | 44 (56.4%) | None | None | mRNA‐1273 | Anti‐S IgG | 28 |
| 9 | Lesny et al. 19 | 2021 | Germany | Cohort | 11 | 2 (18.18) | 14 | 8 | BNT162b2 | Anti‐S IgG | 2 |
| 10 | Duarte et al. 20 | 2021 | Portugal | Cohort | 42 | 21 (50%) | None | none | BNT162b2 | Anti‐S IgG | 21 |
| 11 | Kolb et al. 21 | 2021 | Germany | Case–Control | 32 | 3 (11%) | 78 | 33 (42%) | BNT162b2 | Anti‐RBD IgG | 20 |
| 12 | Zitt et al. 22 | 2021 | Austria | Cohort | 50 | 21 (42%) | None | None | BNT162b2 | Anti‐S IgG | 28 |
Table 2.
Antibody response data in dialysis patients after the second dose of mRNA vaccines
| Author | Year | Country | Type of study | Number of cases | Number of positive cases (%) | Number of the control group | Number of positive controls (%) | Vaccine type | Antibody type | Timing post second dose (days) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Grupper et al. 23 | 2021 | Israel | Case‐Control | 56 | 54 (96%) | 95 | 95 (100%) | BNT162b2 | Anti–RBD IgG | 30 |
| 2 | Speer et al. 8 | 2021 | Germany | Case‐Control | 17 | 14 (82%) | 46 | 46 (100%) | BNT162b2 | Anti‐S1 IgG | 18–22 |
| 3 | Frantzen et al. 24 | 2021 | France | Cohort | 244 | 221 (91%) | None | None | BNT162b2 | Anti‐S IgG | 30 |
| 4 | Simon et al. 25 | 2021 | Austria | Case‐Control | 81 | 74 (91.35%) | 80 | 80 (100%) | BNT162b2 | Anti‐S total | 21 |
| 5 | Jahn et al. 26 | 2021 | Germany | Case‐Control | 72 | 67 (93%) | 16 | 16 (100%) | BNT162b2 | Anti‐S IgG | 14 |
| 6 | Yau et al. 14 | 2021 | Canada | Case‐Control | 72 | 69 (96%) | 35 | 35 (100%) | BNT162b2 | Anti S‐IgG | 14 |
| 7 | Attias et al. 15 | 2021 | France | Cohort | 52 | 43 (82%) | None | None | BNT162b2 | Anti‐S1 IgG | 21 |
| 8 | Schrezenmeier et al. 27 | 2021 | Germany | Case‐Control | 36 | 32 (88.9%) | 44 | 40 (91%) | BNT162b2 | Anti‐RBD IgG | 21 |
| 9 | Agur et al. 28 | 2021 | Israel | Prospective Cohort | 122 | 114 (93.4%) | None | None | BNT162b2 | Anti‐S IgG | 36 |
| 10 | Arevalo et al. 29 | 2021 | Germany | Case‐Control | 44 | 31 (70/5%) | 35 | 35 (100%) | BNT162b2 | Anti‐S1 IgG | 21–28 |
| 11 | Longlune et al. 16 | 2021 | France | Cohort | 77 | 64 (83.11%) | NONE | NONE | BNT162b2 | Anti‐RBD TOTAL | 30 |
| 12 | Strengert et al. 30 | 2021 | Germany | Case‐Control | 81 | 95% | 34 | 34 (100%) | BNT162b2 | Anti‐S1 IgG | 21 |
| 13 | Lacson et al. 31 | 2021 | United States | Cohort | 148 | 130 (88.1%) | None | None | BNT162b2 | Anti‐RBD IgG | ≥14 |
| 14 | Broseta et al. 32 | 2021 | Spain | Cohort | 75 | 69 (92%) | None | None | BNT162b2 | Anti‐RBD IgG | 21 |
| 15 | Garcia et al. 33 | 2021 | United States | Cohort | 416 | 369 (88.6) | None | None | BNT162b2 | Anti‐RBD IgG | 14–28 |
| 16 | Ducloux et al. 34 | 2021 | France | Cohort | 50 | 45 (90%) | None | None | BNT162b2 | Anti‐RBD IgG | 30 |
| 17 | Garcia et al. 33 | 2021 | United States | Cohort | 1316 | 1247 (94.8) | None | None | mRNA‐1273 | Anti‐RBD IgG | 14–28 |
| 18 | Broseta et al. 32 | 2021 | Spain | Cohort | 100 | 98 (98%) | None | None | mRNA‐1273 | Anti‐RBD IgG | 21 |
| 19 | Lacson et al. 31 | 2021 | United States | Cohort | 18 | 17 (94.4%) | None | None | mRNA‐1273 | Anti‐RBD IgG | ≥14 |
| 20 | Espi et al. 18 | 2021 | France | Case‐Control | 92 | 73 (79.35) | 26 | 26 (100%) | BNT162b2 | Anti‐RBD IgG | 10–14 |
| 21 | Espi et al. 32 | 2021 | France | Case‐Control | 75 | 63 (84) | 30 | 30 (100%) | BNT162b2 | Anti‐RBD IgG | 10–14 |
| 22 | Weigert et al. 17 | 2021 | Portugal | Case‐Control | 143 | 130 (90.9%) | 143 | 136 (95.1%) | BNT162b2 | Anti S‐IgG | 21 |
| 23 | Stumpf et al. 35 | 2021 | Germany | Cohort | 936 | 908 (97%) | None | None | mRNA‐1273 | Anti‐S1 IgG | 28 |
| 24 | Stumpf et al. 35 | 2021 | Germany | Cohort | 200 | 176 (88%) | None | None | BNT162b2 | Anti‐S1 IgG | 35 |
| 25 | Clarke et al. 36 | 2021 | England | Cohort | 281 | 248 (88.3%) | None | None | BNT162b2 | Anti S‐IgG | ≥14 |
| 26 | Dulovic et al. 37 | 2021 | Germany | 76 | 72 | 23 | 23 | BNT162b2 | Anti‐RBD IgG | 21 | |
| 27 | Hsu et al. 38 | 2021 | USA | Cohort | 437 | 381 | None | None | BNT162b2 | Anti‐RBD IgG | 14 |
| 28 | Bertrand et al. 39 | 2021 | France | Cohort | 45 | 8 (88.9%) | None | None | BNT162b2 | Anti‐S1 IgG | 14 |
| 29 | Danthu et al. 40 | 2021 | France | Cohort | 78 | 59 (85.5) | 7 | 7 | BNT162b2 | Anti‐S IgG | 14 |
| 30 | Duarte et al. 20 | 2021 | Portugal | Cohort | 42 | 6 (14%) | None | None | BNT162b2 | Anti‐S IgG | 21 |
| 31 | Zitt et al. 22 | 2021 | Austria | Cohort | 48 | 47(97.9%) | None | None | BNT162b2 | Anti‐S IgG | 14 |
| 32 | Paal et al. 41 | 2021 | Germany | Cohort | 179 | 173 (96.6) | 70 | 68 (97.1) | BNT162b2 | Anti‐S IgG | 21 |
| 33 | Giot et al. 42 | 2021 | France | Cohort | 71 | 55 (77%) | None | None | BNT162b2 | Anti‐S IgG | 14 |
| 34 | Dekervel et al. 43 | 2021 | France | Cohort | 66 | 50 | None | None | BNT162b2 | Anti‐S IgG | 14 |
| 35 | Labriola et al. 44 | 2021 | Belgium | Case‐Control | 24 | 19 (79%) | 33 | 28 (85%) | BNT162b2 | Anti‐RBD IgG | 28 |
| 36 | Kolb et al. 21 | 2021 | Germany | Cohort | 32 | 28 (88%) | 78 | 73 | BNT162b2 | Anti‐RBD IgG | 14 |
| 37 | Broseta et al. 18 | 2021 | Spain | Cohort | 78 | 74 (94.9) | None | None | mRNA‐1273 | Anti‐S IgG | 21 |
| 38 | Kaiser et al. 45 | 2021 | Austria | Cohort | 77 | 76 (98.7) | None | None | mRNA‐1273 | Anti‐RBD IgG | 21 |
| 39 | Kaiser et al. 45 | 2021 | Austria | Cohort | 39 | 37 (94.8) | None | None | BNT162b2 | Anti‐RBD IgG | 21 |
| 40 | Yanay et al. 46 | 2021 | Israel | Cohort | 127 | 115 (90.5%) | 132 | 132 | BNT162b2 | Anti‐S IgG | 21–35 |
| 41 | Tylicki et al. 47 | 2021 | Poland | Cohort | 91 | 87 (95.6) | None | None | BNT162b2 | Anti‐S IgG | 14–21 |
Seroconversion rate after the first dose of mRNA vaccines was 36% (95% CI: 0.24–0.47) and 68% (95% CI: 0.45–0.91) in hemodialysis patients and the control group, respectively (Figure 2). While seroconversion rate after the second dose of mRNA vaccines was 86% (95% CI: 0.81–0.91) and 100% (95% CI: 1.00–1.00) in hemodialysis patients and in the control group, respectively (Figure 3). Evaluation of the relationship between vaccine type and seroconversion rate using a meta‐regression model showed no significant differences between any of BNT162b2 and mRNA1273 vaccines and seroconversion rate after two doses of vaccine.
Figure 2.
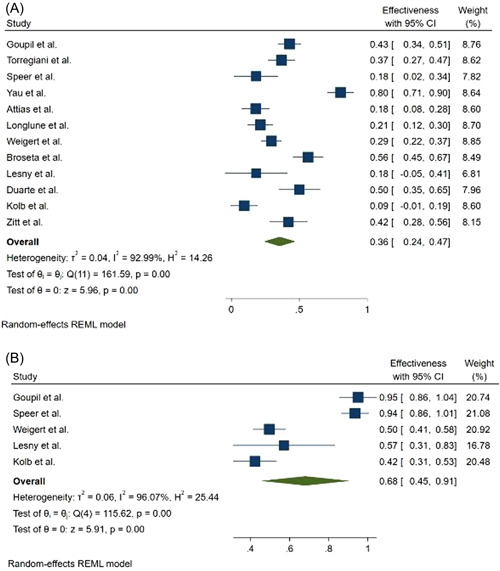
Forest plot for the seroconversion rate after the first dose of mRNA‐based vaccines (A) cases, (B) controls
Figure 3.
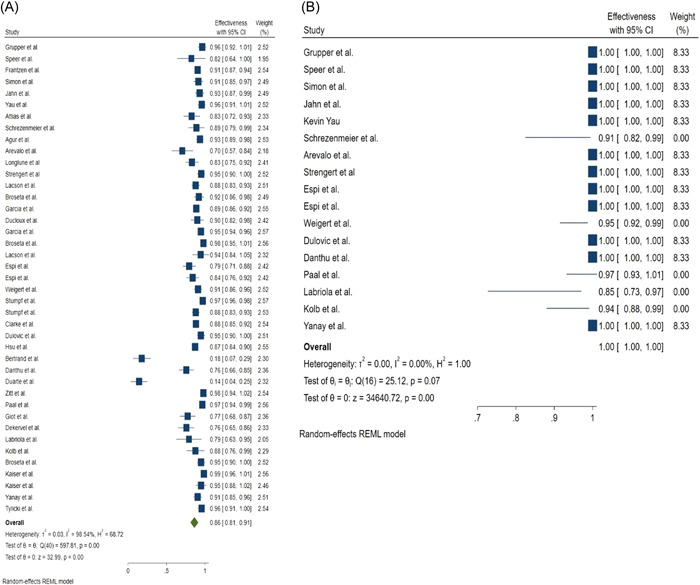
Forest plot for the seroconversion rate after the second dose of mRNA‐based vaccines, (A) Cases, (B) Controls
4. DISCUSSION
The mRNA‐based vaccines showed more than 90% efficacy in preventing COVID‐19 disease. 25 , 48 Patients with severe kidney disease were not present in clinical trials; therefore, the vaccine's efficacy in this high‐risk group has not been evaluated. Compared with the general population, hemodialysis patients have a lower response to hepatitis B and influenza vaccination.
Immunological changes include a distorted Th1/Th2 response, altered function of professional antigen‐presenting cells (APC), and susceptibility of B cells to apoptosis compared with people without kidney disease, making dialysis patients less likely to seroconvert and maintain protective serum titers over time. 31 , 49 After clinical trials and vaccine approval, several studies in dialysis centers examined seroconversion rates following the administration of two doses of mRNA‐based vaccines. The efficacy of vaccination was evaluated 14–30 days post the second injection by quantifying antibodies against spike protein, which shows a strong correlation with neutralizing antibodies.
Our results show that hemodialysis's seroconversion rate after the first dose of mRNA vaccines was 36% (95% CI: 0.24–0.47) and 68% (95% CI: 0.45–0.91) in patients and the control group, respectively. While seroconversion rate after the second dose of mRNA vaccines was 86% (95% CI: 0.81–0.91) and 100% (95% CI: 1.00–1.00) in hemodialysis patients and the control group, respectively.
The results of this meta‐analysis show that the seroconversion rate is low after the first dose of the vaccine, and the administration of the second dose should not be delayed. Although the seroconversion rate after the second dose in hemodialysis patients is lower than in the control group, it is very promising for hemodialysis patients.
Our results showed that mRNA‐based vaccines induce comparable seroconversion rates in hemodialysis patients and healthy controls. Our results indicate that the mRNA platform can be used to improve the immunogenicity of vaccines against other pathogens in hemodialysis patients.
The COVID‐19 mRNA‐based vaccine immunogenicity in hemodialysis patients is much greater than that of influenza and hepatitis B vaccines immunogenicity.
Several factors can contribute to the higher immunogenicity of mRNA‐based vaccines compared with the previous vaccines among hemodialysis patients. First, the vaccine platform is different; second, mRNA‐based vaccines’ efficacy has been studied in hemodialysis patients during the pandemic. It is possible that in some individuals, the combination of natural immunity after infection and vaccine‐generated immunity positively impacts the vaccine efficacy. 15 , 50 However, in some studies, initial/baseline infection or history of the previous infection of COVID‐19 has been actively monitored in the study population; these people were excluded from the study or located in a separate group. 15 , 27 , 28 , 51
The limitations of this study include: the small sample size, and also our study includes articles that assessed the efficacy of mRNA‐based vaccines by antibody titer. seroconversion and antibody titer is an easy method to evaluate the immunological response to vaccination, but it is not equivalent to complete protection. 52 Also, the antibody levels required to protect against COVID‐19 have not yet been determined. 53 However, to assess vaccine responses, it is recommended to assess both humoral and cellular responses. 54 However, due to limited data on vaccine‐mediated cellular immunity, this study focused on investigating humoral immune responses after vaccination.
Although the seroconversion rate is high, many studies have reported that the antibody level of hemodialysis patients after being vaccinated with the COVID‐19 vaccine is lower than that of the control group; as a result, a shorter period of immune protection can be assumed. Therefore, it is necessary to periodically assess the antibody levels of hemodialysis patients at short intervals and renew their vaccination when necessary.
5. CONCLUSION
Although the immune response of hemodialysis patients to the second dose of SARS‐CoV‐2 mRNA vaccine is very promising, the seroconversion rate of dialysis patients is lower than healthy controls. As a result, it is necessary to pay more attention to the vaccination programs of this population, periodically assess the antibody levels of hemodialysis patients at short intervals and renew their vaccination when necessary.
AUTHOR CONTRIBUTIONS
Shahab Falahi: Conceptualization; data curation; formal analysis; supervision; writing – original draft; writing – review & editing. Hojjat Sayyadi: Conceptualization; data curation; formal analysis; methodology; software. Azra Kenarkoohi: Conceptualization; data curation; formal analysis; project administration; supervision; writing – original draft; writing – review & editing.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
TRANSPARENCY STATEMENT
The lead author Azra Kenarkoohi affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Falahi S, Sayyadi H, Kenarkoohi A. Immunogenicity of COVID‐19 mRNA vaccines in hemodialysis patients: systematic review and meta‐analysis. Health Sci Rep. 2022;5:e874. 10.1002/hsr2.874
DATA AVAILABILITY STATEMENT
Data sharing does not apply to this article as no datasets were generated or analyzed during the current study.
REFERENCES
- 1. Falahi S, Abdoli A, Kenarkoohi A. Claims and reasons about mild COVID‐19 in children. New Microbes New Infect. 2021;41:100864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gheysarzadeh A, Sadeghifard N, Safari M, et al. Report of Five Nurses Infected With Severe Acute Respiratory Syndrome Coronavirus 2 During Patient Care: Case Series. Elsevier; 2020:100694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Falahi S, Kenarkoohi A. Sex and gender differences in the outcome of patients with COVID‐19. J Med Virol. 2020;93:151‐152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sadeghifar J, Jalilian H, Momeni K, et al. Outcome evaluation of COVID‐19 infected patients by disease symptoms: a cross‐sectional study in Ilam province, Iran. BMC Infect Dis. 2021;21(1):1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Abdoli A, Taghipour A, Pirestani M, et al. Infections, inflammation, and risk of neuropsychiatric disorders: the neglected role of “co‐infection”. Heliyon. 2020;6(12):e05645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Falahi S, Kenarkoohi A. Transmission routes for SARS‐CoV‐2 infection: review of evidence. New Microbes New Infect. 2020;38:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Falahi S, Kenarkoohi A. COVID‐19 reinfection: prolonged shedding or true reinfection? New Microbes New Infect. 2020;38:100812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Speer C, Göth D, Benning L, et al. Early humoral responses of hemodialysis patients after COVID‐19 vaccination with BNT162b2. Clin J Am Soc Nephrol. 2021;16(7):1073‐1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kenarkoohi A, Maleki M, Ghiasi B, et al. Prevalence and clinical presentation of COVID‐19 infection in hemodialysis patients. J Nephropathol. 2022;11(1):1‐6. [Google Scholar]
- 10. Kliger AS, Silberzweig J. COVID‐19 and dialysis patients: unsolved problems in early 2021. J Am Soc Nephrol. 2021;32(5):1018‐1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Miskulin DC, Combe C. mRNA COVID‐19 vaccine for people with kidney failure: hope but prudence warranted. Clin J Am Soc Nephrol. 2021;16(7):996‐998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goupil R, Benlarbi M, Beaubien‐Souligny W, et al. Short‐term antibody response after 1 dose of BNT162b2 vaccine in patients receiving hemodialysis. CMAJ. 2021;193(22):E793‐E800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Torreggiani M, Blanchi S, Fois A, Fessi H, Piccoli GB. Neutralizing SARS‐CoV‐2 antibody response in dialysis patients after the first dose of the BNT162b2 mRNA COVID‐19 vaccine: the war is far from being won. Kidney Int. 2021;99(6):1494‐1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yau K, Abe KT, Naimark DM, et al. The Humoral Response to the BNT162b2 Vaccine in Hemodialysis Patients. JAMA . 2021;4(9): e2123622. [Google Scholar]
- 15. Attias P, Sakhi H, Rieu P, et al. Antibody response to the BNT162b2 vaccine in maintenance hemodialysis patients. Kidney Int. 2021;99(6):1490‐1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Longlune N, Nogier MB, Miedougé M, et al. High immunogenicity of a messenger RNA‐based vaccine against SARS‐CoV‐2 in chronic dialysis patients. Nephrol Dial Transplant. 2021;36(9):1704‐1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Weigert A, Bergman M‐L, Gonçalves L, et al. Longitudinal analysis of antibody responses to the Pfizer BNT162b2 vaccine in patients undergoing maintenance hemodialysis. Front Med . 2021;8:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Broseta JJ, Rodríguez‐Espinosa D, Soruco E, Maduell F. Weekly seroconversion rate of the mRNA‐1273 SARS‐CoV‐2 vaccine in haemodialysis patients. Nephrol Dial Transplant. 2021;36(9):1754‐1755. [DOI] [PubMed] [Google Scholar]
- 19. Lesny P, Anderson M, Cloherty G, et al. Immunogenicity of a first dose of mRNA‐or vector‐based SARS‐CoV‐2 vaccination in dialysis patients: a multicenter prospective observational pilot study. J Nephrol. 2021;34:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Duarte RA, Roldão M, Figueiredo C, et al. Humoral response to BNT162b2 mRNA Covid19 vaccine in peritoneal and hemodialysis patients: a comparative study. medRxiv. 2021. [DOI] [PMC free article] [PubMed]
- 21. Kolb T, Fischer S, Müller L, et al. Impaired immune response to SARS‐CoV‐2 vaccination in dialysis patients and in kidney transplant recipients. Kidney. 2021;3602(9):1491‐1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zitt E, Davidovic T, Schimpf J, et al. The safety and immunogenicity of the mRNA‐BNT162b2 SARS‐CoV‐2 vaccine in hemodialysis patients. Front Immunol. 2021;12:2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Grupper A, Sharon N, Finn T, et al. Humoral response to the pfizer BNT162b2 vaccine in patients undergoing maintenance hemodialysis. Clin J Amer Soc Nephrol. 2021;16(7):1037‐1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Frantzen L, Cavaillé G, Thibeaut S, El‐Haik Y. Efficacy of the BNT162b2 mRNA COVID‐19 vaccine in a haemodialysis cohort. Nephrol Dial Transplant. 2021;36(9):1756‐1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Simon B, Rubey H, Treipl A, et al. Haemodialysis patients show a highly diminished antibody response after COVID‐19 mRNA vaccination compared with healthy controls. Nephrol Dial Transplant. 2021;36(9):1709‐1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jahn M, Korth J, Dorsch O, et al. Humoral response to SARS‐CoV‐2‐vaccination with BNT162b2 (pfizer‐biontech) in patients on hemodialysis. Vaccines. 2021;9(4):360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schrezenmeier E, Bergfeld L, Hillus D, et al. Immunogenicity of COVID‐19 tozinameran vaccination in patients on chronic dialysis. Front Immunol. 2021;12:690698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Agur T, Ben‐Dor N, Goldman S, et al. Antibody response to mRNA SARS‐CoV‐2 vaccine among dialysis patients—a prospectivecohort study. Nephrol Dial Transplant. 2021;36(7):1347‐1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rincon‐Arevalo H, Choi M, Stefanski A‐L, et al. Impaired humoral immunity to SARS‐CoV‐2 BNT162b2 vaccine in kidney transplant recipients and dialysis patients. Science Immunology. 2021;6(60):eabj1031. [DOI] [PubMed] [Google Scholar]
- 30. Strengert M, Becker M, Ramos GM, et al. Cellular and humoral immunogenicity of a SARS‐CoV‐2 mRNA vaccine in patients on haemodialysis. EBioMedicine. 2021;70:103524‐ 103525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lacson E, Jr. , Argyropoulos CP, Manley HJ, et al. Immunogenicity of SARS‐CoV‐2 vaccine in dialysis. J Am Soc Nephrol. 2021;32:2735‐2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Broseta JJ, Rodríguez‐Espinosa D, Rodríguez N, et al. Humoral and cellular responses to mRNA‐1273 and BNT162b2 SARS‐CoV‐2 vaccines administered to hemodialysis patients. Am J Kidney Dis. 2021;78(4):571‐581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Garcia P, Anand S, Han J, et al. COVID19 vaccine type and humoral immune response in patients receiving dialysis. medRxiv. 2021. [DOI] [PMC free article] [PubMed]
- 34. Ducloux D, Colladant M, Chabannes M, Yannaraki M, Courivaud C. Humoral response after BNT162b2 mRNA COVID‐19 vaccination in patients on haemodialysis depends on immune status. Clin Kidney J. 2021;14(10):2266‐2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Stumpf J, Siepmann T, Lindner T, et al. Humoral and cellular immunity to SARS‐CoV‐2 vaccination in renal transplant versus dialysis patients: a prospective, multicenter observational study using mRNA‐1273 or BNT162b2 mRNA vaccine. Lancet Reg Health Eur. 2021;9:100178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Clarke CL, Prendecki M, Dhutia A, et al. Longevity of SARS‐CoV‐2 immune responses in hemodialysis patients and protection against reinfection. Kidney Int. 2021;99(6):1470‐1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dulovic A, Strengert M, Ramos GM, et al. Diminishing immune responses against variants of concern in dialysis patients four months after SARS‐CoV‐2 mRNA vaccination. Emerg Infec Dis. 2022; 28(4):743‐750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hsu CM, Weiner DE, Manley HJ, et al. Seroresponse to SARS‐CoV‐2 vaccines among maintenance dialysis patients over six months. CJASN . 2022;17(3):403‐413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bertrand D, Hamzaoui M, Lemée V, et al. Antibody and T cell response to SARS‐CoV‐2 messenger RNA BNT162b2 vaccine in kidney transplant recipients and hemodialysis patients. J Am Soc Nephrol. 2021;32(9):2147‐2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Danthu C, Hantz S, Dahlem A, et al. Humoral response after SARS‐CoV‐2 mRNA vaccination in a cohort of hemodialysis patients and kidney transplant recipients. J Am Soc Nephrol. 2021;32(9):2153‐2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Paal M, Arend FM, Lau T, et al. Antibody response to mRNA SARS‐CoV‐2 vaccines in haemodialysis patients. Clin Kidney J. 2021;14(10):2234‐2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Giot M, Fourié T, Lano G, et al. Spike and neutralizing antibodies response to COVID‐19 vaccination in haemodialysis patients. Clin Kidney J. 2021;14(10):2239‐2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dekervel M, Henry N, Torreggiani M, et al. Humoral response to a third injection of BNT162b2 vaccine in patients on maintenance haemodialysis. Clin Kidney J. 2021;14:2349‐2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Labriola L, Scohy A, Van Regemorter E, et al. Immunogenicity of BNT162b2 SARS‐CoV‐2 vaccine in a multicenter cohort of nursing home residents receiving maintenance hemodialysis. Am J Kidney Dis. 2021;78(5):766‐768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kaiser RA, Haller MC, Apfalter P, Kerschner H, Cejka D. Comparison of BNT162b2 (Pfizer–BioNtech) and mRNA‐1273 (moderna) SARS‐CoV‐2 mRNA vaccine immunogenicity in dialysis patients. Kidney Int. 2021;100(3):697‐698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yanay NB, Freiman S, Shapira M, et al. Experience with SARS‐CoV‐2 BNT162b2 mRNA vaccine in dialysis patients. Kidney Int. 2021;99(6):1496‐1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tylicki L, Biedunkiewicz B, Dąbrowska M, et al. Humoral response to SARS‐CoV‐2 vaccination promises to improve the catastrophic prognosis of hemodialysis patients as a result of COVID‐19: the COViNEPH project. Pol Arch Med Wewn. 2021;131(9):797‐801. [DOI] [PubMed] [Google Scholar]
- 48. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA‐1273 SARS‐CoV‐2 vaccine. N Engl J Med. 2021;384(5):403‐416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Abdoli A, Falahi S, Kenarkoohi A. COVID‐19‐associated opportunistic infections: a snapshot on the current reports. Clin Exp Med. 2021;23:1‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Falahi S, Kenarkoohi A. Host factors and vaccine efficacy: implications for COVID‐19 vaccines. J Med Virol. 2022; 94(4):1330‐ 1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mirnejad R, Fallahi S, Kiani J, et al. Epidemic assessment of bacterial agents in osteomyelitis and their antibiotic resistance pattern determination. J Biol Sci. 2008;8(2):478‐481. [Google Scholar]
- 52. Anand S, Montez‐Rath ME, Han J, et al. Antibody response to COVID‐19 vaccination in patients receiving dialysis. J Am Soc Nephrol. 2021;32(10):2435‐2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wei J, Stoesser N, Matthews PC, et al. Antibody responses to SARS‐CoV‐2 vaccines in 45,965 adults from the general population of the United Kingdom. Nat Microbiol. 2021;6(9):1140‐1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Shachor‐Meyouhas Y, Hussein K, Szwarcwort‐Cohen M, et al. Single BNT162b2 vaccine dose produces seroconversion in under 60 s cohort. Vaccine. 2021;39(47):6902‐6906. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing does not apply to this article as no datasets were generated or analyzed during the current study.


