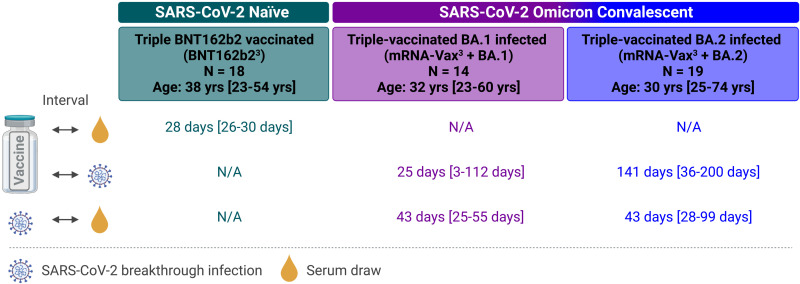
Fig. 1. Cohorts and sampling.
Serum samples were drawn from three cohorts: individuals triple-vaccinated with BNT162b2 that were SARS-CoV-2-naïve at the time of sampling (BNT162b23, green), and from individuals vaccinated with three doses of mRNA COVID-19 vaccine (BNT162b2/mRNA-1273 homologous or heterologous regimens) who subsequently had a breakthrough infection with Omicron either at a time of BA.1 dominance (November 2021 to January 2022; mRNA-Vax3 + BA.1, purple) or at a time of BA.2 dominance (March to May 2022; mRNA-Vax3 + BA.2, blue). For convalescent cohorts, relevant intervals between key events such as the most recent vaccination, SARS-CoV-2 infection, and serum isolation are indicated. All values specified as median-range. The age/sex composition of cohorts is further detailed in Tables S1 to S3. Data for the reference cohorts BNT162b23 and mRNA-Vax3 + BA.1 were previously published (10), except for newly generated BA.2.12.1 neutralization data. N/A, not applicable; Schematic was created with BioRender.com