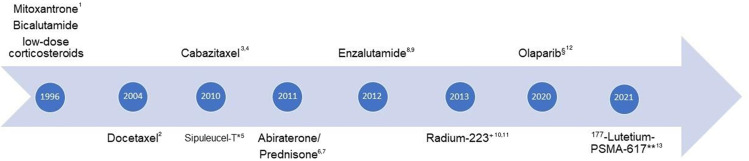
Figure 1.
Timeline of treatments for mCRPC (year of reported positive pivotal trial). 1,2Tannock, IF et al. N Engl J Med 2004; 3De Bono, J et al. Lancet 2010, 4Oudard, S et al. J Clin Oncol 2017, 5Kantoff, P.W. et al. N Engl J Med. 2010; 6De Bono, J et al. N Engl J Med. 2011, 7Ryan, CJ et al. N Engl J Med. 2013, 8Scher, HI et al. N Engl J Med 2012; 9Beer, TM et al. N Engl J Med 2014, 10Parker, C et al. N Engl J Med. 2013, 11Nilsson S et al. Ann Oncol. 2016; 12De Bono, J et al. N Engl J Med 2020;13Sartor O et al. N Engl J Med. 2021. *Approval EMA withdrawn, not available in Europe; +symptomatic, bone only, LN<3cm, visceral mets. excluded; approval §EMA: BRCA1,2 FDA: HRD panel PROfound, Rucaparib (FDA only); **FDA only approval, not EMA.