Figure 2. OspA-based vaccines in recent clinical trials.
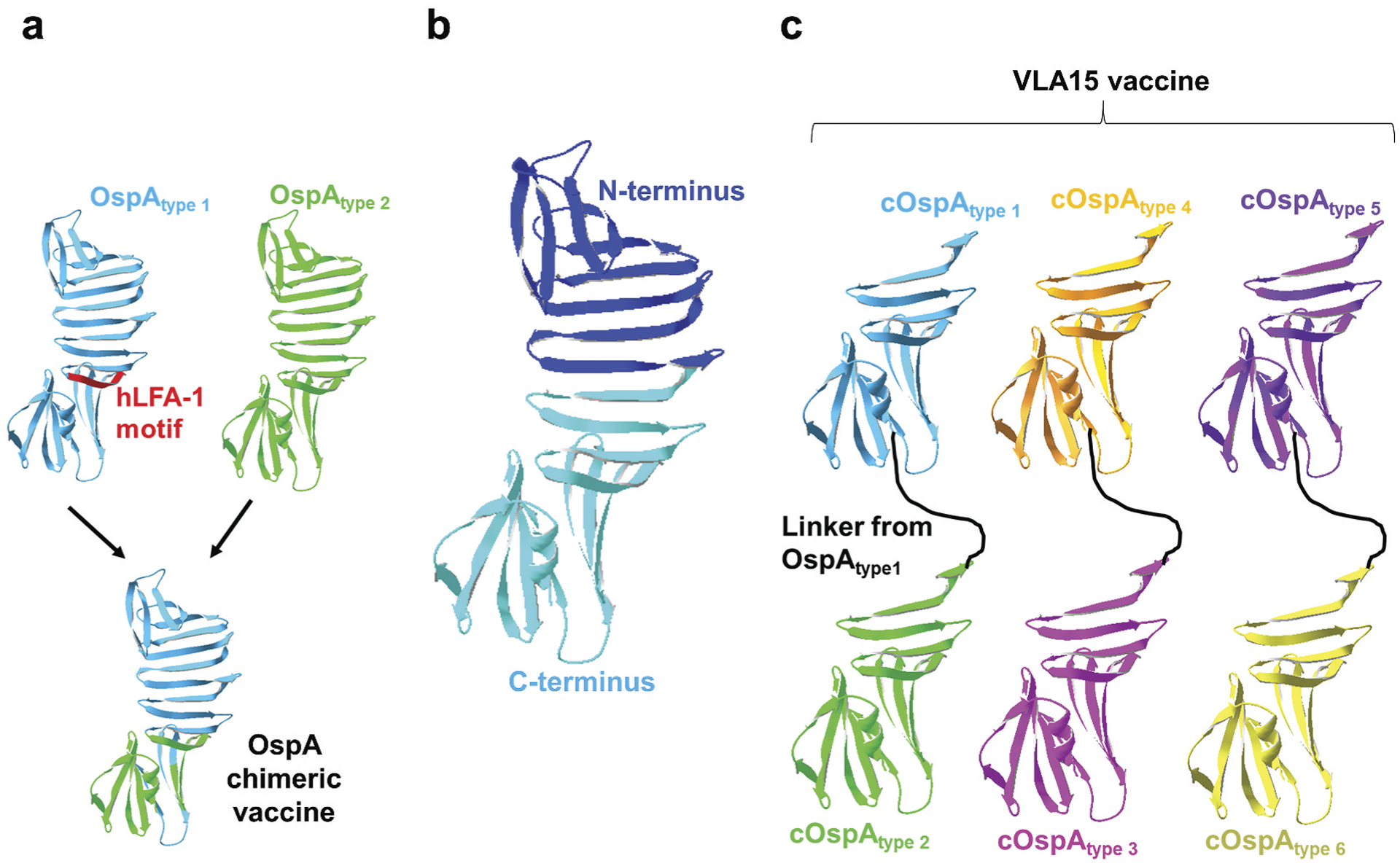
(a) Shown is the OspA chimeric vaccine from OspA variants of Borrelia burgdorferi (for OspA type 1) and B. afzelii (for OspA type 2) with the elimination of the autoimmune motif on OspA type 1 (human LFA-1 motif; hLFA-1 motif). (b) N-terminal (dark blue) and C-terminal (light blue) regions of OspA type 1 comprising amino acids 1 to 272 and 131 to 273, respectively, as defined in [133]. (c) Shown is the VLA15 vaccine that contains C-terminal OspA variants from any two of the six most commonly found human infectious OspA types of Lyme borreliae (OspA type 1 and 2, 3 and 4, and 5 and 6) connected with a linker from the sequences of OspA type 1.
