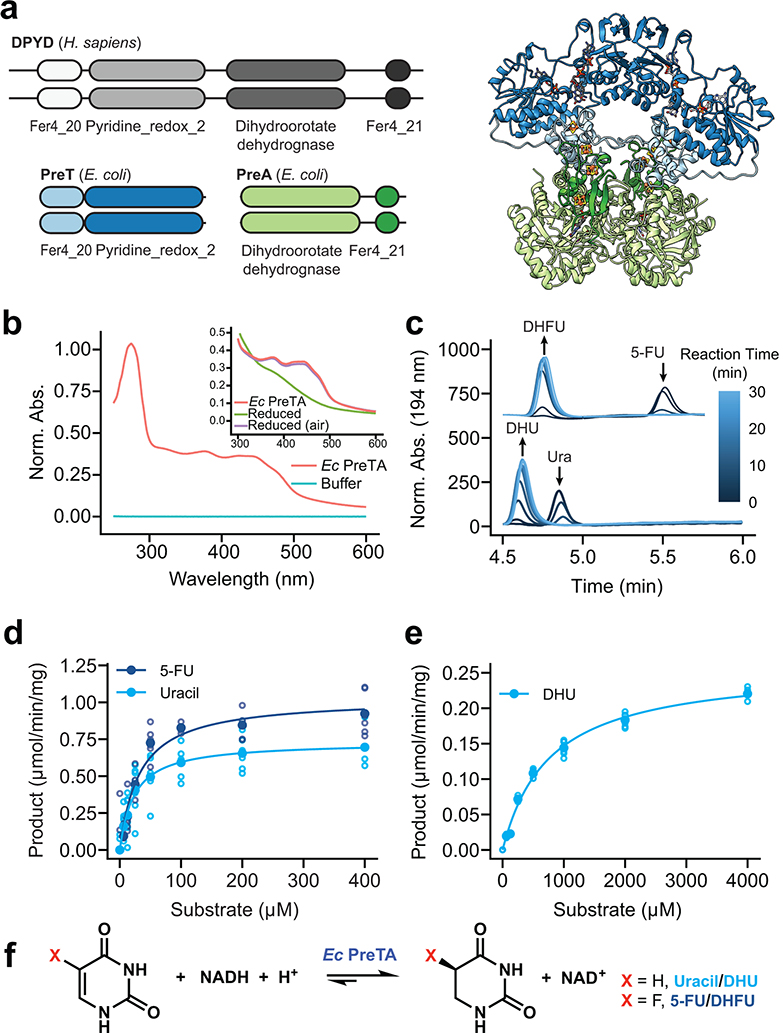
Fig. 3|. E. coli PreTA more rapidly catalyzes the reduction of pyrimidines.
(a) A cartoon schematic shows the domain architecture of the homodimeric H. sapiens DPYD and heterotetrameric E. coli PreTA, as well as a E. coli PreTA homology model (Swiss-Model99). Protein backbone colors are indicated by the schematic, cofactors are colored by heteroatom. (b) UV-visible absorption spectra indicate heterologously expressed E. coli PreTA has characteristic peaks due to 4Fe-4S and flavin cofactors. Inset shows reduction of flavin cofactors and reoxidation upon exposure to air. (c) In vitro enzyme assays analyzed by high pressure liquid chromatography (HPLC) show conversion of uracil and 5-FU to their reduced forms over time. (d-e) Michaelis-Menten analysis of NADH cofactor consumption and accumulation in vitro enzyme assays in the reductive (d) and oxidative (e) directions display a marked difference in the ability for E. coli PreTA to catalyze the oxidative reaction. Solid points represent the mean of the hollow circles, n=6 replicates over 2 different enzyme preparations. (f) Schematic of the chemical reaction catalyzed by E. coli PreTA.