Abstract
p38 MAPKs are key regulators of cellular adaptation to various stress stimuli, however, their role in mediating erythrocyte cell death and hemolysis is largely unknown. We hypothesized that activation of erythrocyte p38 MAPK is a common event in the stimulation of hemolysis, and that inhibition of p38 MAPK pathways could mitigate hemolysis in hemoglobinopathies. We exposed human erythrocytes to diamide-induced oxidative stress or to hypoosmotic shock in the presence or absence of p38 MAPK inhibitors (SCIO469, SB203580, CMPD1) and used immunoblotting to determine MAPK activity and to identify possible downstream effectors of p38 MAPK. We also evaluated the impact of p38 MAPK inhibitors on stress-induced hemolysis or hypoxia-induced sickling in erythrocytes from mouse models of sickle cell disease. We found that human erythrocytes express conventional MAPKs (MKK3, p38 MAPK, MAPKAPK2) and identified differential MAPK activation pathways in each stress condition. Specifically, p38 MAPK inhibition in diamide-treated erythrocytes was associated with decreased phosphorylation of Src tyrosine kinases and Band 3 protein. Conversely, hypoosmotic shock induced MAPKAPK2 and RSK2 phosphorylation, which was inhibited by SCIO469 or CMPD1. Relevant to hemoglobinopathies, sickle cell disease was associated with increased erythrocyte MKK3, p38 MAPK, and MAPKAPK2 expression and phosphorylation as compared with erythrocytes from healthy individuals. Furthermore, p38 MAPK inhibition was associated with decreased hemolysis in response to diamide treatments or osmotic shock, and with decreased erythrocyte sickling under experimental hypoxia. These findings provided insights into MAPK-mediated signaling pathways that regulate erythrocyte function and hemolysis in response to extracellular stressors or human diseases.
Keywords: p38 MAPK, Hemolysis, Erythrocyte, Sickle cell disease, Oxidative stress
1. Introduction
Mammalian mitogen-activated protein kinases (MAPKs) are a family of serine/threonine kinases known for their vital roles in mediating cellular responses to various extracellular stimuli including osmotic shock, endotoxins, cytokines, radiation (ultraviolet, gamma rays), cold shock, and reactive oxygen species [1-5]. Key members of this family are the four isoforms of p38 MAPKs (p38 MAPKα, p38 MAPKβ, p38 MAPKγ, and p38 MAPKδ), of which canonical p38 MAPKα signaling pathways have been reported in a wide range of human diseases including cardiovascular dysfunction, inflammation, cancer, Alzheimer’s disease, atherosclerosis, and rheumatoid arthritis [6,7]. The p38 MAPK signaling cascade can be induced by G-protein-coupled receptors (GPCRs) upon ligand binding or extracellular stimulus [8]. This nexus involves p38 MAPK activation via phosphorylation of a TGY motif in the activation loop by other MAPKs, most commonly the dual specificity mitogen-activated protein kinase kinase 3 or 6 (MKK3, MKK6) [9]. Depending on the stimulus, p38 MAPK signaling may involve concomitant translocation to specific cellular compartments, such as the plasma membrane or the nucleolus [10], and activation of downstream effectors, specifically a subfamily of MAPK-activated protein kinases (MAP-KAPKs) including MAPKAPK2 (MK2). The involvement of p38 MAPK pathways in the pathogenesis of inflammatory diseases (e.g. rheumatoid arthritis) [11-13], certain types of cancers [14], and recently reported COVID-19 [15] has triggered the investigation of therapeutics targeted at inhibiting p38 MAPK or its downstream messengers (e.g. MK2) [12,16].
Several studies reported that inhibition of p38 MAPK pathways via pharmacological intervention or genetic mutations in mice significantly interfered with normal or stress erythropoiesis [17-23]. Collectively, these studies suggested that p38 MAPKs play a pivotal role in the regulation of erythropoiesis and anemia via mechanisms that may involve erythropoietin [19,24]. For example, p38 MAPK promoted the proliferation of erythroid progenitor cells [25], mediated γ globin (fetal hemoglobin) induction [26], induced erythroblast differentiation [21], and erythroblast enucleation under stress erythropoiesis [17]. Furthermore, p38 MAPKα knockdown in mice resulted in defective erythropoiesis and anemia [19]. Despite their established role in regulating erythroid cell proliferation, differentiation, and maturation, little is known about the action of p38 MAPKs in mature erythrocytes with regard to extracellular stress stimuli or in the setting of hemolytic diseases. A previous study reported the activation of p38 MAPK upon exposure of erythrocytes to hyperosmotic shock [27]. This type of stress resulted in cell shrinkage and translocation of phosphatidylserine to the outer layer of the cell membrane that was partially inhibited by p38 MAPK inhibitors. This study concluded that activation of p38 MAPK pathways was associated with erythrocyte-programmed cell death (eryptosis). Additional studies revealed that p38 MAPK inhibition in vitro was protective against eryptosis or hemolysis induced by antimicrobial and antifungal agents (triclosan, anidulafungin, manumycin A) [28-30], antitumor compounds (allicin, bioymifi) [31,32], and occupational pollutants (nickel chloride) [33]. Erythrocytes from patients with sickle cell disease (SCD) have been shown to express other members of the MAPK family (MEK1/2 and ERK1/2), whose activation by epinephrine promoted adhesion to endothelial cells; a key event in the pathogenesis of vaso-occlusion in SCD [34].
In this report, we sought to characterize erythrocyte MAPK expression and activation under stress conditions that promote hemolysis including oxidative stress, osmotic shock, and hemolytic disorders (SCD, thalassemia). We hypothesized that activation of erythrocyte p38 MAPK is a key and common event in the stimulation of hemolysis, and that inhibition of these pathways could mitigate the risk of hemolysis in various conditions, such as hemolytic disorders, anemia, hemodialysis, and ex vivo preservation of red blood cells. To verify a functional role for MAPKs in molecular pathways that result in hemolysis, we subjected human erythrocytes to diamide-induced oxidative stress or hypoosmotic shock in the presence or absence of MAPK inhibitors and quantified the expression of membrane-bound proteins from the p38 MAPK signaling cascade (e.g., MKK3, MK2) and the rates of hemolysis. We further determined the expression of erythrocyte MAPKs in thalassemia and SCD, and tested the effect of p38 MAPK inhibitors on hypoxia-induced dehydration and sickling in SCD.
2. Materials and methods
2.1. Human subjects
This study was conducted under regulations applicable to all human subject research supported by federal agencies including institutional review board (IRB) approval from Vitalant Research Institute in Denver, and the University of Colorado Anschutz Medical Campus. Human erythrocytes used in this study were obtained by whole blood collection (≤50 mL) into EDTA vacutainer tubes from 27 healthy individuals including 19 women and 8 men. Average age was 38.5 ± 9.8. All donors read the Donor Acknowledgment and Consent information and sign the consent form under Vitalant’s IRB protocol number 20181957. Donor eligibility was confirmed using Vitalant Research Screening Record with a standard blood donor health questionnaire including questions about medications (aspirin, antibiotic, corticosteroids), pregnancy, drug abuse, recent blood donation/transfusion, infectious diseases, and blood disorders. Inclusion criteria was age ≥ 18 years. Exclusion criteria included RBC disorders that could impact RBC MAPK expression (homozygotes to sickle cell disease, thalassemia, spherocytosis). For MAPK studies in SCD, whole blood (5 mL) from individuals with sickle cell disease was collected from 5 patients (three females and two males; average age 15 ± 1.4 years) who were consented under the University of Colorado Anschutz Medical Campus IRB protocol number 20–0505. Inclusion criteria were individuals of all ages with homozygous sickle cell disease or sickle beta zero thalassemia who were willing to consent to the study. Exclusion criteria were < 10 kg body weight, refusal or decisionally challenged individuals.
2.2. Experimental mice
All experiments were approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh IACUC Protocol numbers 15106093 and 16109265. Animal husbandry was carried out according to the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The mice used for this study were from two strains of transgenic models of sickle cell disease including Hbatm1Paz Hbbtm1Tow Tg(HBA-HBBs)41Paz/J (Berkeley model) [35], and B6;129-Hbatm1(HBA)Tow Hbbtm2(HBG1,HBB*)Tow/Hbbtm3(HBG1,HBB)Tow/J (Towne model) [36]. Homozygous mice from both strains express human α-globin and sickle β-globin (HbS, human sickle hemoglobin) chains, which render their erythrocytes susceptible to sickling under hypoxia similar to human erythrocytes from patients with sickle cell disease. We also evaluated p38 MAPK expression in a transgenic mouse model of β-thalassemia (B6.129P2-Hbb-b1tm1Unc Hbb-b2tm1Unc/J) [37]. All mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). Procedures performed on mice involved exsanguination under a deep plane of inhalation anesthesia using cardiac puncture followed by cervical dislocation. Blood collected into heparinized microtubes from male and female mice (3–6 months old) was centrifuged (1500 xg, 10 min, 18 °C) and erythrocytes were isolated by removing plasma and buffy coat followed by two washes with phosphate buffered saline (PBS).
2.3. Erythrocyte stress assays in the presence of MAPK inhibitors
Erythrocytes used in this study were assayed on the day of collection or within 3 days after collection. In this case, whole blood samples were stored at 4 °C. Prior to all treatments, aliquots of whole blood were washed three times (1500 xg, 10 min, 18 °C) in PBS to obtain erythrocyte pellets. Validation studies with a hematological analyzer and light microscopy to evaluate possible contamination of erythrocyte pellets by residual white blood cells (WBCs) or platelets, demonstrated contaminating cells were undetected or negligible (0.005% WBCs and 0.05% platelets at most). Human erythrocytes were subjected to two stress hemolysis assays in the presence or absence of p38 MAPK inhibitors including SCIO469 [16], CMPD1 [38] (p38 MAPKα inhibitor), and SB203580 hydrochloride (a water-soluble p38 MAPKα/β inhibitor). All p38 inhibitors were from Tocris Bioscience, (Minneapolis, USA). Additional inhibitors included MK2 inhibitor IV (MAPKAPK2 inhibitor) and Src Kinase Inhibitor 1 (Src inhibitor) were purchased from Cayman Chemical (Ann Harbor, USA). In all experiments, stock solutions were prepared in double-distilled water (SCIO469, SB203580) or dimethyl sulfoxide (DMSO; CMPD1, MK2 inhibitor IV, Src Kinase Inhibitor 1). Final concentrations of the inhibitors in erythrocyte suspensions ranged from 10 μmol/L to 100 μmol/L after dilution (1:1000) into erythrocyte suspensions in PBS (diamide) or pink test (hypoosmotic shock) buffer (a Bis-Tris buffer containing 25 mmol/L sodium chloride, 70 mmol/L Bis-Tris, and 135 mmol/L glycerol; pH 6.6). Control samples were treated in the same manner as test samples using the solvent of each drug. For the hemolysis assays, hemoglobin levels were determined using the Drabkin’s method [39]. Incubation times were optimized for the types of assays being investigated (i.e., hemolysis versus pathway activation for Western blotting).
2.3.1. Hypoosmotic shock
Erythrocyte osmotic fragility was determined by a modified pink test [40] as described before [41]. Briefly, washed erythrocyte concentrates were added to pink test buffer in the presence of selected MAPK inhibitors or vehicle controls. The average hematocrit measured 1.6 ± 0.2%. Samples were gently mixed and incubated at room temperature for 2 h (pathway activation) or 4 h (hemolysis), after which they were spun at 1500 xg for 10 min at 18 °C. Percent osmotic hemolysis was determined using the equation , in which Hbosmotic corresponds to the cell-free hemoglobin and Hbtotal corresponds to the total amount of hemoglobin in each sample. In this assay, we did not determine the concertation of cell-free hemoglobin in erythrocytes incubated in isotonic buffer (control) as this condition does not induce hemolysis under the tested conditions.
2.3.2. Diamide-induced oxidative stress
Oxidative injury was induced by diamide, an oxidizing compound reported to induce oxidative stress via activation of erythrocyte spleen tyrosine kinase (Syk), which promotes membrane injury and vesiculation via Band 3 (erythrocyte anion exchanger-1) phosphorylation and dimerization [42,43]. Erythrocyte suspensions (3.5 ± 0.5% in PBS) were incubated in the presence or absence of diamide (2 mmol/L) at 37 °C for 4 h (pathway activation) or 16 h (hemolysis). Percent oxidative hemolysis was determined by , where HbDiamdie refers to the cell-free hemoglobin of diamide-treated erythrocytes. Hbcontrol refers to the cell-free hemoglobin of untreated erythrocytes, and Hbtotal refers to the total amount of hemoglobin in each sample.
2.4. Quantification of protein expression in erythrocyte membranes
Following erythrocyte stress assays, membrane fractions (red blood cell ghosts) were prepared using a modified protocol originally described by Dodge et al. [44] as detailed in Supplemental Methods. Total protein measurement of each sample was quantified using Pierce BCA protein assay kit (thermo scientific). These data were used to load equal amounts of protein onto the gel across all samples. Erythrocyte membrane-bound protein expression and activation (phosphorylation) were determined by immunoblot analyses of selected proteins including phosphorylated and total MKK3, p38 MAPK, MAPKAPK2, RSK2, Src, Band 3, and actin for normalization. A comprehensive list of all antibodies used for immunoblotting and procedures is available in Supplemental Methods.
2.5. Evaluation of p38 MAPK inhibition on hypoxia-induced sickling in sickle cell disease
Washed erythrocyte concentrates from mouse models of SCD (Berkeley and Towne strains) were diluted 1:25 with PBS and treated with SCIO469 (100 μmol/L) or vehicle control (0.1% double distilled water). After treatments, all samples were incubated for 15 min at 22 °C with mild agitation prior to hypoxic incubation (37 °C) in a hypoxic chamber. Initially, samples were incubated on a plate shaker under mild hypoxia (15% O2, 5% CO2) for 15 min followed by gradual oxygen depletion to 5% and further incubation under these conditions for 30 min. After incubation, all samples were fixed under hypoxia (0.1% glutaraldehyde) before preparation of erythrocyte smears and staining with WG-16 (Sigma Aldrich, St. Louis, USA). This step was necessary to prevent changes in erythrocyte shapes due to reoxygenation. At least two images (about 100 cells each) were used for the evaluation of percent sickling in each sample. Evaluation was conducted under light microscopy on de identified samples to avoid bias. Scoring was determined by counting erythrocytes with normal phenotype (biconcave) and abnormal shapes (defined as sickle and other combined) and calculating the percentage of abnormal cells on the total number of cells.
2.6. Statistical analysis
All statistical tests were performed using GraphPad Prism version 9.3.1. The type of analysis was determined by normality tests (D’Agostino-Pearson method sample size of N ≥ 8 and Shapiro-Wilk test normality test for N < 8). Datasets (e.g., protein expression, percent hemolysis) that passed normality tests were analyzed using Prism’s parametric mixed-effects ANOVA analysis with Geisser-Greenhouse correction and multiple comparison test. Dunnett test was used in experiments that evaluated the effect of a single MAPK inhibitor using the vehicle control as reference. In cases where more than one MAPK inhibitor was evaluated (Western blotting analyses), the mean effect of each inhibitor or untreated controls was compared to the stressor alone (diamide, hypoosmotic) using Fisher’s LSD test. Differences between two independent groups (healthy versus thalassemic/sickle cell disease erythrocytes) were determined by unpaired t-test, whereas paired t-test were used to evaluate the effect of SCIO469 on erythrocyte sickling. In datasets that did not pass normality tests, non-parametric tests were used including Kruskal-Wallis with Dunn’s multiple comparison test, and Mann-Whitney for paired comparisons. In all analyses, significance was determined by p ≤ 0.05.
3. Results
3.1. Inhibition of erythrocyte p38 MAPK is protective against oxidative and osmotic hemolysis in vitro
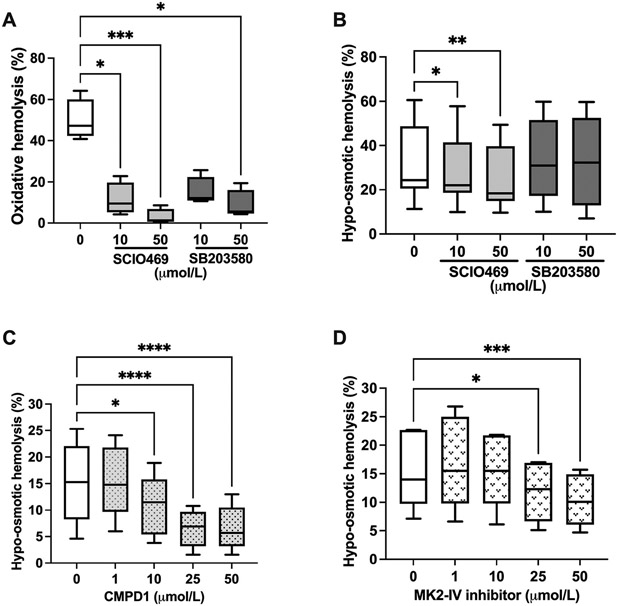
To verify a role for p38 MAPKs in molecular signaling that promote hemolysis, human erythrocytes were subjected to diamide-induced oxidative stress or hypoosmotic shock in the presence or absence of p38 MAPK inhibitors (Fig. 1). Erythrocyte incubation with diamide (2 mmol/L, 16 h, 37 °C) resulted in about 50% hemolysis, which was significantly inhibited in a dose dependent manner by SCIO469 and SB203580 (Fig. 1A). For example, at 10 μmol/L, hemolysis measured 11.5 ± 8.0% (SCIO469; p = 0.027) as compared to 49.9 ± 10% in vehicle control (DMSO 0.1%). SCIO469 significantly (p = 0.02 at 10 μmol/L, and p = 0.0035 at 50 μmol/L) decreased hemolysis that was induced by hypoosmotic shock (Fig. 1B) in a dose dependent manner, whereas SB203580 at the tested concentrations (10 μmol/L and 50 μmol/L) had no effect on osmotic hemolysis.
Fig. 1.
Inhibiting p38 MAPK in human erythrocytes reduces hemolysis in response to oxidative or hypoosmotic stress. A. Percent oxidative hemolysis following erythrocyte incubation with diamide (2 mmol/L, 16 h) in the presence of the p38 MAPK inhibitors SCIO469 and SB203580 at selected micromolar concentrations. N = 4; 1 experiment. *p < 0.05, ***p = 0.0004 by Kruskal-Wallis test. B. Percent osmotic hemolysis following erythrocyte incubation in pink test buffer (4 h) in the presence of the p38 MAPK inhibitors SCIO469 and SB203580 at selected micromolar concentrations. N = 8 and N = 6 for SCIO469 and SB203580 treatments, respectively; 3 independent experiments. *p = 0.02, **p = 0.0035. C. Percent osmotic hemolysis following erythrocyte incubation in pink test buffer (4 h) in the presence of the p38 MAPKα inhibitor CMPD1 at selected micromolar concentrations. N = 5; one experiment. *p = 0.019, ****p < 0.0001. D. Percent osmotic hemolysis following erythrocyte incubation in pink test buffer (4 h) in the presence of MAPKAPK2 inhibitor (MK2 inhibitor IV) at selected micromolar concentrations. N = 5; 1 experiment. *p = 0.011, ***p = 0.0007. Data are presented as box and whiskers (median and min to max). B–D: p values were obtained by mixed-effects ANOVA analysis and Dunnett’s multiple comparison test. In all analyses, the mean effect of each inhibitor was compared to the vehicle control.
Given the high selectivity of SCIO469 to p38α, we hypothesized that this isoform is specifically involved in erythrocyte volume regulation under osmotic stress. We therefore tested the action of CMPD1, a non-ATP competitive inhibitor of the p38α-MAPKAPK2 signaling axis, in our subsequent evaluations of osmotic hemolysis. Similar to SCIO469, CMPD1 significantly (p = 0.019 at 10 μmol/L, and p < 0.0001 at 25 and 50 μmol/L) decreased osmotic hemolysis as compared to vehicle control (DMSO 0.1%; Fig. 1C). Because CMPD1 acts through MAPKAPK2, we verified whether a MAPKAPK2-specific inhibitor, MK2 inhibitor IV, can also protect against osmotic hemolysis. As illustrated in Fig. 1D, MAPKAPK2 inhibition was associated with decreased osmotic hemolysis at concentrations of 25 μmol/L (p = 0.011) and 50 μmol/L (p = 0.0007) compared to vehicle control.
3.2. p38 MAPK signaling pathways in diamide-treated erythrocytes involve Band 3 and Src proteins
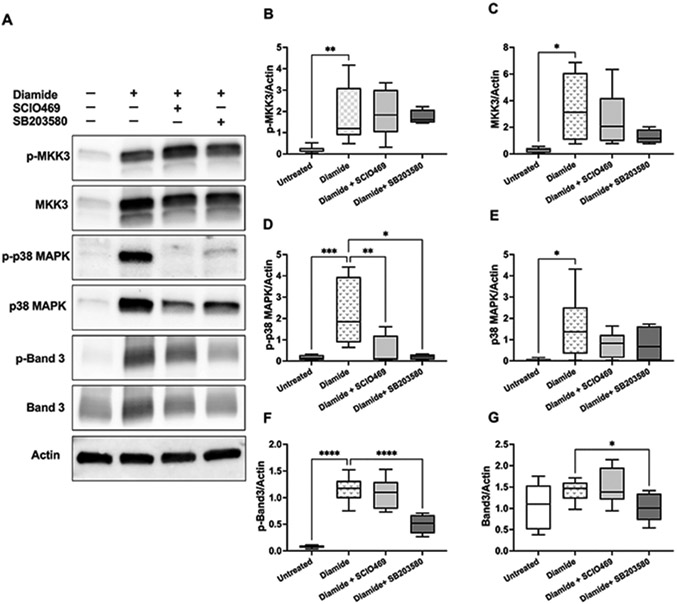
Incubation of erythrocytes with diamide (2 mmol/L, 2 h) resulted in increased erythrocyte membrane association of total and phosphorylated MKK3, p38 MAPK, and Band 3 (Fig. 2A). p38 MAPK inhibitors (SCIO469 and SB203580; both at 50 μmol/L) had no significant effect on membrane-associated phospho-MKK3 and total MKK3 (Fig. 2B-C). Conversely, both drugs demonstrated strong and significant inhibition of p38 MAPK phosphorylation in response to diamide but had no significant impact on total p38 MAPK levels as compared with diamide alone (Fig. 2D-E). Next, we verified whether p38 MAPK activation in response to diamide involves MAPKAPK2 activation and found no expression of phosphorylated MAPKAPK2 in erythrocytes ghosts (Supplemental Fig. S1). Diamide treatments resulted in Band 3 tyrosine phosphorylation (Y359), which was significantly inhibited by SB203580 (p < 0.0001) (Fig. 2F). SB203580 treatment was also associated with decreased (p = 0.0205) total Band 3 expression compared to diamide treatment alone (Fig. 2G).
Fig. 2.
Activation of p38 signaling pathways in response to diamide-induced oxidative stress. Western blotting analyses of human erythrocyte membranes following incubations with diamide (2 mmol/L, 4 h) in the presence or absence of p38 MAPK inhibitors SCIO469 (50 μmol/L) or SB203580 (50 μmol/L). A. Representative immunoblots of phosphorylated (p-) and total MKK3, p38 MAPK, Band 3, and actin. Quantified protein expressions (normalized to actin) are shown in B–G. B–E: N = 8 for SCIO469 and N = 4 for SB203580 treatments. F–G: N = 8 for SCIO469 and N = 6 for SB203580 treatments. B. Phospho-MKK3; **p = 0.0026. C. Total MKK; *p = 0.0193. D. Phospho-p38 MAPK; *p = 0.0131, **p =0.009, ***p = 0.0009. E. Total p38 MAPK; *p = 0.0157. F. Phospho-Band 3; ****p < 0.0001. G. Total Band 3; *p = 0.0205. Data were collected from 4 independent experiments and are presented as box and whiskers (median and min to max). B, D: p values were obtained by Kruskal–Wallis test. C,E-G: p values were obtained by mixed-effects ANOVA analysis and Fisher’s LSD test. In all analyses, the mean effect of each inhibitor or untreated controls was compared to diamide treatment.
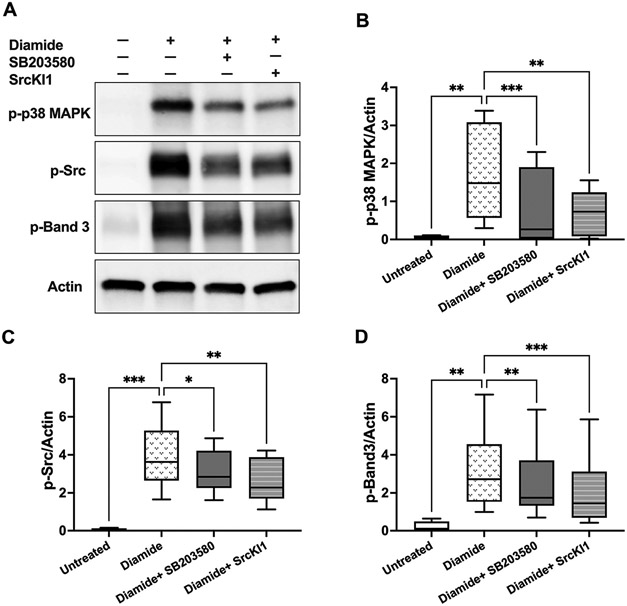
Because p38 MAPK inhibition was associated with attenuated Band 3 tyrosine phosphorylation in diamide-treated erythrocytes, we asked whether these signaling cascades involve interactions with tyrosine kinases, such as cellular Src. As shown in Fig. 3, diamide treatments (2 mmol/L, 4 h) resulted in the phosphorylation of p38 MAPK, Src, and Band 3 proteins in erythrocyte ghosts. Addition of Src kinase inhibitor 1 (SrcKI1, 500 nmol/L) significantly decreased the phosphorylation of p38 MAPK (p = 0.007), Src (p = 0.0064), and Band 3 (p = 0.0007) in response to diamide. Similar effects were observed with SB203580 (10 μmol/L), although it was less effective in preventing Band 3 phosphorylation compared with the Src inhibitor.
Fig. 3.
Possible interactions between p38 MAPK and Src proteins in diamide-treated erythrocytes: Western blotting analyses of human erythrocyte membranes following erythrocyte incubations with diamide (2 mmol/L, 4 h) in the presence or absence of p38 MAPK inhibitor (SB203580 10 μmol/L) or Src inhibitor (SrcKI1; 500 nmol/L). A. Representative immunoblots of phosphorylated (p-) p38 MAPK, Src, Band 3, and total actin. Quantified protein expressions (normalized to actin) are shown in B–D. N = 8. B. phospho-p38 MAPK; **p < 0.008, ***p = 0.0008. C. phospho-Src; *p = 0.0446, **p = 0.0064, ***p = 0.0003. D. phospho-Band 3; **p < 0.003, ***p = 0.0007. All p values were obtained by mixed-effects ANOVA analysis and Fisher’s LSD test. Data were collected from 3 independent experiments and are presented as box and whiskers (median and min to max). In all analyses, the mean effect of each inhibitor or untreated controls was compared to diamide treatment.
3.3. Hypoosmotic shock activates erythrocyte p38 MAPK signaling pathways that involve MAPKAPK2 and RSK2
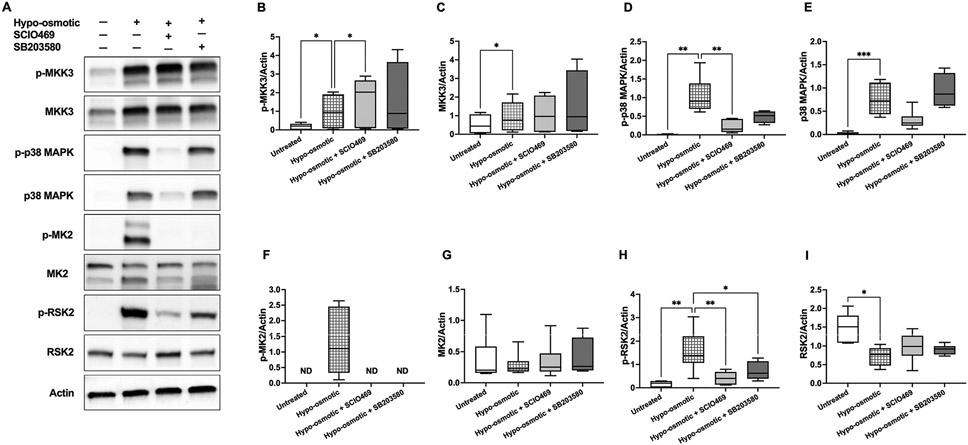
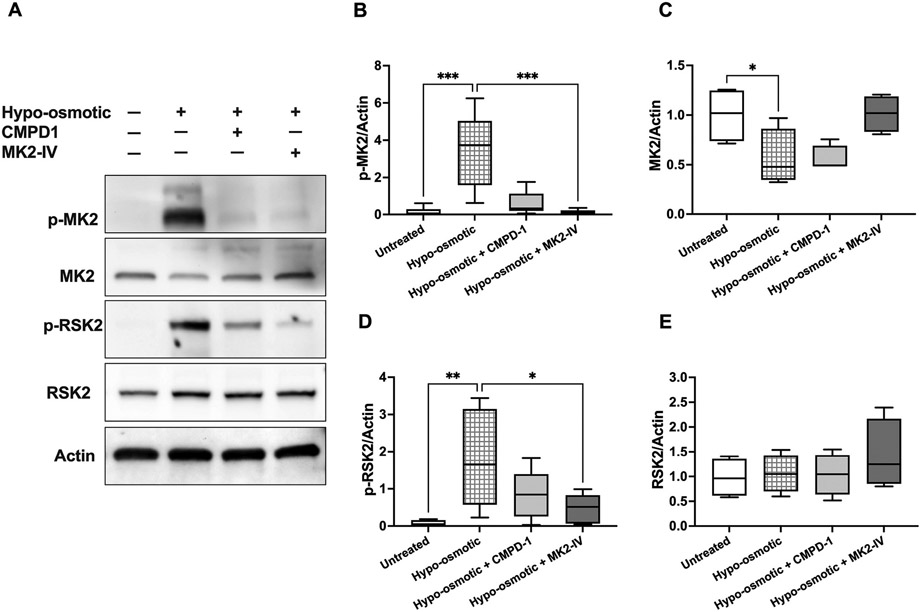
Exposure of human erythrocytes to hypoosmotic shock (2 h, pink test buffer) resulted in increased membrane association and phosphorylation of MKK3 and p38 MAPK (Fig. 4A-E). p38 MAPK inhibition by SCIO469 (50 μmol/L) was associated increased MKK3 phosphorylation (about 1.6-fold change of the mean; p = 0.0481; Fig. 4B) and with decreased expression of phosphorylated (about 5-fold change of the mean; p = 0.0048) p38 MAPK (Fig. 4D). p38 MAPK inhibition by SB203580 (50 μmol/L) was less effective than SCIO469 in preventing p38 MAPK phosphorylation and not effective in preventing p38 MAPK membrane association (Fig. 4D-E). p38 MAPK activation was associated with phosphorylation of MAPKAPK2 (p-MK2), which was completely inhibited by both p38 MAPK inhibitors (Fig. 4F). Of note, the MAPKAPK2 immunoblots (Fig. 4 and MK2) show two bands (around 45 kDa and 50 kDa) that likely represent the two known MAPKAPK2 isoforms generated by alternative splicing. Our search for stress kinases that act downstream of p38 MAPK and MAPKAPK2 signaling identified RSK2, a ribosomal S6 kinase (RSK) that belongs to a family of four serine/threonine kinases [45]. Compared with untreated erythrocytes, hypoosmotic shock was associated with significant expression (p = 0.0072) of phosphorylated RSK2, which was inhibited by SCIO469 (p = 0.0072) and SB203580 (p = 0.0313) (Fig. 4H).
Fig. 4.
Activation of p38 signaling pathways in response to hypoosmotic stress. Western blotting analyses of human erythrocyte membranes following erythrocyte incubations in pink test buffer (2 h) in the presence or absence of p38 MAPK inhibitors SCIO469 (50 μmol/L) or SB203580 (50 μmol/L). A. Representative immunoblots of phosphorylated (p-) and total MKK3, p38 MAPK, MAPKAPK2, RSK2, and actin. Quantified protein expressions (normalized to actin) are shown in B–I. B. Phospho-MKK3; *p < 0.05. C. Total MKK3; *p = 0.048. D. Phospho-p38 MAPK; **p < 0.005. E. Total p38 MAPK; ***p = 0.0005. F. Phospho-MAPKAPK2 (p-MK2). G. Total MAPKAPK2 (MK2). H. Phospho-RSK2; *p = 0.0313, **p = 0.0072. I. Total RSK2; p = 0.0157. B–G: N = 6 for SCIO469 and N = 4 for SB203580 treatments. H–I: N = 6. All p values were obtained by mixed-effects ANOVA analysis and Fisher’s LSD test, except for panel E, where Kruskal–Wallis test was used. Data were collected from 4 independent experiments and are presented as box and whiskers (median and min to max). In all analyses, the mean effect of each inhibitor or untreated controls was compared to hypoosmotic treatment.
To test the hypothesis that RSK2 acts downstream of MAPKAPK2, we verified whether MAPKAPK2 inhibitors (CMPD-1 and MK2 inhibitor IV) modulate erythrocyte RSK2 phosphorylation under hypoosmotic stress (Fig. 5). MAPKAPK2 phosphorylation was reduced in the presence of CMPD-1 (25 μ mol/L; p = 0.0511) or MK2 inhibitor IV (25 μmol/L; p = 0.0006) (Fig. 5B). The concentration of each inhibitor (25 μmol/L) was chosen based on its efficacy to inhibit osmotic hemolysis as shown in Fig. 1C-D. A significant decrease in RSK2 phosphorylation was observed in the presence of MK2 inhibitor IV (about 3.9-fold change of the mean; p = 0.0167) (Fig. 5D). No significant changes were observed in total RSK2 expression among the tested groups.
Fig. 5.
Effect of p38 MAPKα and MAPKAPK2 inhibitors on erythrocyte RSK2 activation and expression in response to hypoosmotic stress. Western blotting analyses of human erythrocyte membranes following erythrocyte incubations in pink test buffer (2 h) in the presence or absence of p38 MAPKα inhibitor (CMPD1; 25 μmol/L) or MAPKAPK2 inhibitor (MK2-IV; 25 μmol/L). A. Representative immunoblots of phosphorylated (p-) and total MAPAPK2 (MK2), RSK2, and actin. Quantified protein expressions (normalized to actin) are shown in B–E. B. Phospho-MAPKAPK2 (p-MK2); ***p ≤ 0.0006 by Kruskal–Wallis test. N = 7. C. Total MAPKAPK2 (MK2); *p = 0.045 by Kruskal–Wallis test. N = 4. D. Phospho-RSK2; *p = 0.0167, **p = 0.0094 by mixed-effects ANOVA analysis and Fisher’s LSD test. N = 7. E. Total RSK2. N = 4. Data were collected from 3 independent experiments and are presented as box and whiskers (median and min to max). In all analyses, the mean effect of each inhibitor or untreated controls was compared to hypoosmotic treatment.
3.4. Differential erythrocyte MAPK expression in thalassemia and sickle cell disease
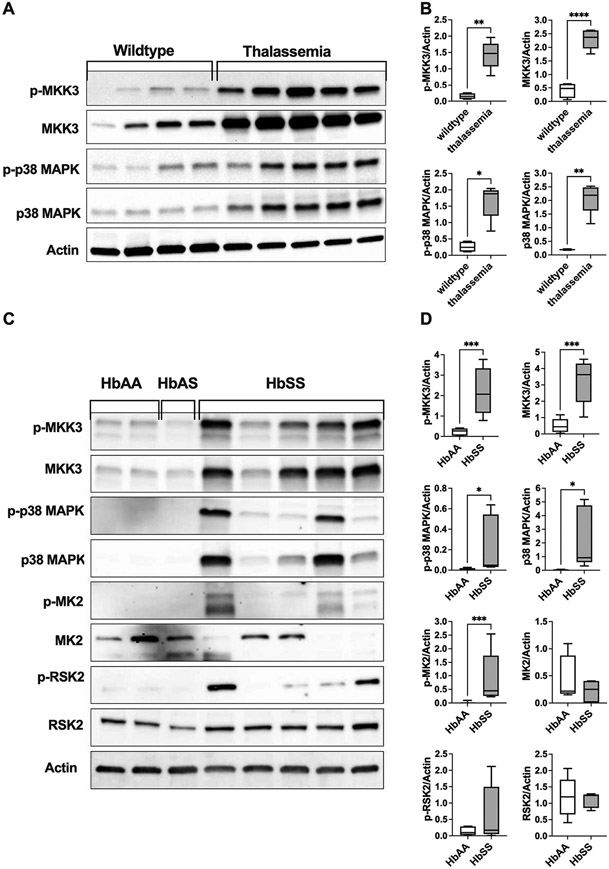
To verify relevance to hemolytic diseases, we asked whether erythrocyte p38 MAPK signaling pathways are upregulated in thalassemia and sickle cell disease (Fig. 6). Erythrocyte membranes from a mouse model of β-thalassemia (B6.129P2-Hbb-b1tm1Unc Hbb-b2tm1Unc/J) expressed significantly (all p values <0.02) higher levels of phosphorylated and total MKK3 and p38 MAPK as compared with wildtype controls (Fig. 6A-B). Similarly, we observed increased expression of phosphorylated MKK3, p38 MAPK, MAPKAPK2, total MKK3, and total p38 MAPK in erythrocyte membranes from patients with sickle cell disease (HbSS) as compared to erythrocytes from healthy individuals (HbAA) or an individual with sickle cell trait (HbAS) (Fig. 6C-D).
Fig. 6.
Differential expression of erythrocyte MAPK proteins in thalassemia and sickle cell disease. Western blotting analyses of erythrocyte membranes from a mouse model of β-thalassemia (B6.129P2-Hbb-b1tm1Unc Hbb-b2tm1Unc/J) or from patients with sickle cell disease. A. Representative immunoblots of phosphorylated (p-) and total MKK3, p38 MAPK, and actin in erythrocyte ghost from thalassemic mice and wildtype controls (C57BL/6J). B. Quantified protein expressions (normalized to actin) are shown for phospho-MKK3, total MKK3, phospho-p38 MAPK, and total p38 MAPK. N = 5 (thalassemic mice) and N = 4 (wildtypes). *p = 0.0159 by Mann–Whitney test; **p < 0.002 and ****p < 0.0001 by unpaired t-test. C. Representative immunoblots of phosphorylated (p-) and total MKK3, p38 MAPK, MAPAPK2 (MK2), RSK2, and actin in erythrocyte membranes from healthy volunteers (HbAA), a male donor with sickle cell trait (HbAS), and patients with sickle cell disease (HbSS). D. Quantified protein expressions (normalized to actin) are shown for phospho-MKK3, total MKK3, phospho-p38 MAPK, total p38 MAPK, phospho-MAPAPK2 (MK2), total MK2, phospho-RSK2, and total RSK2. N = 5 (HbSS) and N = 8 (HbAA). *p < 0.05, ***p ≤ 0.0008 by unpaired t-test, except for p-MK2 where Mann–Whitney test was used. Data were collected from 2 (panel B) and 3 (Panel D) independent experiments and are presented as box and whiskers (median and min to max).
3.5. p38 MAPK inhibition attenuates hypoxia-induced erythrocyte dehydration and sickling in mouse models of sickle cell disease
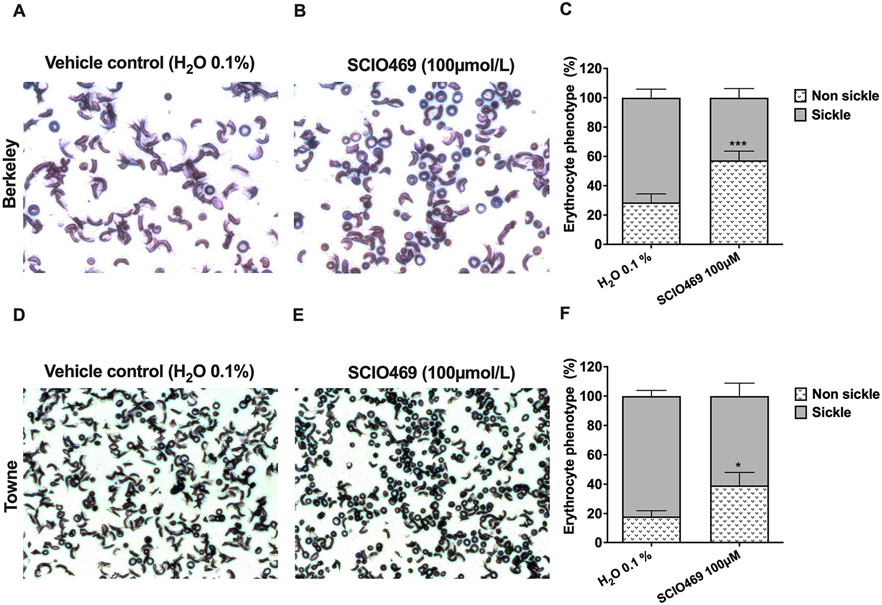
Given the role p38 MAPKs play in regulation of cellular responses to osmotic stress, we asked whether inhibition of p38 MAPK pathways in sickle cell disease alters erythrocyte responses to hypoxia-induced dehydration and sickling. Hypoxic incubation of murine erythrocytes from two sickle cell disease model strains (Berkeley and Towne) resulted in sickling phenotypes in over 70% of the cells (Fig. 7). Under the same experimental conditions, p38 MAPK inhibition by SCIO469 (100 μmol/L) significantly decreased the rate of erythrocyte sickling from 71.4 ± 5.8% to 42.7 ± 6.3% in cells from Berkeley mice (p = 0.0006; Fig. 7B-C) and from 81.4 ± 9.5% to 60.8 ± 8.8% in cells from Towne mice (p = 0.0247; Fig. 7E-F).
Fig. 7.
Effect of p38 inhibitor (SCIO469) on hypoxia-induced erythrocyte dehydration and sickling in mouse models of sickle cell disease. Erythrocytes from two strains of sickle cell disease including Hbatm1PazHbbtm1TowTg(HBA-HBBs)41Paz/J (Berkeley; N = 4; Panels A–C) and B6;129-Hbatm1(HBA)TowHbbtm2(HBG1,HBB*)Tow/Hbbtm3(HBG1,HBB)Tow/J (Towne; N = 4; Panels D–F) were incubated under hypoxia in the presence of a p38 MAPK inhibitor (SCIO469, 100 μmol/L) or with vehicle control (H2O 0.1%) and later evaluated for erythrocyte phenotype (percent sickle and non-sickle) as described in Methods. Representative images are shown for vehicle controls (A, D) and SCIO469-treated erythrocytes (B, E). Bar graphs (C and F) represent the percent (mean with SEM) of sickle erythrocytes overlaid with non-sickle. Data were collected from 2 independent experiments. ***p = 0.0006 (C) and *p = 0.0247 (D) by paired t-test (non-sickle phenotype SCIO469 versus vehicle control).
4. Discussion
This study revealed that human and mouse erythrocytes express known members of the MAPK family and provided evidence of MAPK signaling pathways that promote hemolysis under conditions of experimental oxidative stress, osmotic shock, and hemolytic diseases. These observations suggest that in addition to the essential role MAPKs play in erythroid cell differentiation and enucleation [17,21], MAPK activity is preserved in mature erythrocytes, and like other eukaryotic cells, such activity is associated with cellular responses to stress. We chose to explore p38 MAPK for its regulation of multiple cellular functions and its reported involvement in human diseases including COVID19 [6,15,46]. In support of a hypothesis that p38 MAPK mediates erythrocyte stress responses to various stimuli, we demonstrated that erythrocyte exposure to oxidative or osmotic stress resulted in p38 MAPK phosphorylation and that pharmacological inhibition of p38 MAPK was associated with significant decreases in oxidative and osmotic hemolysis. Under the tested conditions, hypoosmotic shock was associated with the strongest activation of p38 MAPK pathways as compared with hyper-osmotic shock or diamide (Supplemental Fig. S1).
Pharmacological inhibition of p38 MAPK by SCIO469 or SB203580 was highly effective in preventing oxidative hemolysis in response to diamide treatments (Fig. 1A). In support of this finding, diamide treatments were associated with phosphorylation of erythrocyte p38 MAPK, which was abrogated by the action of each inhibitor. p38 MAPK inhibitors did not modify the phosphorylation of MKK3 suggesting that this kinase is upstream of p38 MAPK (Fig. 2). Interestingly, we have not observed any changes in MAPKAPK2 (MK2) expression (Supplemental Fig. S1) suggesting that this downstream effector of p38 MAPK was not activated in response to diamide treatments. Tyrosine phosphorylation of Band 3 has been associated with detrimental changes to erythrocyte membrane and vaso-occlusion in sickle cell disease [47]. This mechanism has been attributed to the action of Syk and other erythrocyte tyrosine kinases. Our data suggest that p38 MAPK may be involved in the signaling cascades that promote Band 3 tyrosine phosphorylation under oxidative stress via cross talk with Src proteins (Fig. 3). The nature of the interaction is not clear and warrants further investigation.
This study highlighted the role MAPKs play in regulating cellular responses to osmotic stress. Specific to erythrocytes, we demonstrated that pharmacological inhibition of p38 MAPK or MAPKAPK2 was associated with decreased osmotic hemolysis (Fig. 1B-D) and decreased expression of phosphorylated MAPKAPK2 and RSK2 (Figs. 4, 5). These data revealed that under the tested conditions, p38 MAPK signaling involved the activation of MAPKAPK2 and RSK2. Although MAPKAPK2 has been reported to be directly activated by p38 MAPK [48], its role in mediating stress responses and hemolysis in erythrocytes has not been established. Furthermore, our data suggest that RSK2 acts downstream of MAPKAPK2 under osmotic stress. To our knowledge, a functional role for RSK2 in erythrocytes has not been reported. Mutations in the RSK2 gene have been associated with Coffin-Lowry Syndrome [49], whereas recent studies reported the role that RSK2 plays in the etiology of cancer [50]. Of note, our genome-wide association studies of hemolysis in stored erythrocytes from 13,403 blood donors revealed that single nucleotide polymorphisms (SNPs) in the MAPKAPK5 gene were significantly (genome-wide p < 5 × 10−8) associated with hypoosmotic hemolysis [51]. MAPKAPK5, also known as p38-regulated/activated protein kinase (PRAK), is an atypical MAPK that can be activated by conventional MAPKs (i.e., substrates of MAPKKs including p38 MAPK) and atypical MAPKs (e.g., ERK3/ERK4) [52,53].
A prior study associated p38 MAPK activation with eryptosis in human erythrocytes exposed to hyper-osmotic shock [27]. In agreement with this study, we demonstrated that hyperosmotic shock (high sucrose; 500 mmol/L) promoted p38 MAPK phosphorylation that was concomitant with Band 3 tyrosine phosphorylation (Supplemental Fig. S1). Similar to diamide treatments (Fig. 2), p38 MAPK inhibition by SB203580 significantly (p = 0.0479) reduced the expression of phosphorylated Band 3 (Supplemental Fig. S2) suggesting that p38 MAPK is involved the modification of Band 3 (i.e., dimer and tetramer formations) under hyper-osmotic shock.
A key observation of clinical relevance is the increased expression of phosphorylated and membrane-associated MKK3 and p38 MAPK in erythrocytes from a mouse model of β-thalassemia and from patients with sickle cell disease. The latter also expressed increased levels of phosphorylated MAPKAPK2 (Fig. 6). Furthermore, p38 MAPK inhibition in erythrocytes from two mouse models of sickle cell disease was associated with decreased sickling under experimental hypoxia (Fig. 7). Taken together, these findings further support a role for p38 MAPK in the regulation of cellular responses to osmotic stress and dehydration under hypoxia.
Of note, only erythrocyte membrane fractions were analyzed in this study to minimize interreference by free hemoglobin. Based on these analyses and tested conditions, we hypothesize that p38 MAPK signal transduction combined activation (phosphorylation) by MKK3 and translocation to the membrane. This is supported by the evidence that p38 MAPK expression (total and phosphorylated) in unstimulated erythrocyte membranes (i.e., untreated controls) was negligible compared to oxidative or osmotic stress stimulated erythrocytes. Interestingly, MAPKAPK2 and RSK2 total protein expression in unstimulated erythrocyte membranes was comparable or higher than that of osmotic stress-treated cells (Figs. 4, 5). These data suggest that these kinases are located in the vicinity of the cytoskeleton and the membrane, in which decreased membrane expression in osmotic shock-treated erythrocytes may have resulted from membrane injury and hemolysis.
This study has several limitations including the large variability in the rates of MAPK protein phosphorylation and membrane expression across individual samples (Figs. 2-5). This variation may reflect interindividual differences in erythrocyte susceptibility to oxidative and osmotic stress, which may have influenced the activation of MAPK pathways, the effectiveness of the tested inhibitors, and our statistical analyses. This assumption is supported by the observation of interindividual variability in oxidative and osmotic hemolysis (Fig. 1), and by our previous studies in large blood donor population (N = 13,403) that identified genetic and biologic modifiers of hemolysis [51,54]. Despite those differences, we observed good reproducibility in MAPK protein expression and phosphorylation across to all tested individuals. Additionally, the proposed signaling pathways were identified using pharmacological inhibition of MAPKs and other proteins under ex vivo conditions, however, these observations are supported by our evaluations in erythrocytes from sickle cell disease patients and thalassemic mice. The concentrations of the p38 MAPK inhibitors used in this study ranged from 10 to 100 μmol/L. In most cases, cellular responses were observed at concentrations of 10 μmol/L or higher, which is comparable to studies that used SB203580 [55,56] or SCIO469 [14,57]. However, it is possible that at higher micromolar concentrations, some of the outcomes we observed may have been related to off-target effects of these inhibitors. For this reason, we validated our data using additional inhibitors (e.g., CMPD1, MK2 inhibitor IV) when possible.
Another limitation is that although SB203580 and SCIO469 belong to a class of ATP-competitive inhibitors, we observed differences in their action with regard to p38 MAPK inhibition or protection against osmotic hemolysis. This may be partially explained by differences in drug selectivity to the p38 MAPK isoforms, in which SCIO469 is highly selective for p38 MAPKα over p38 MAPKβ, whereas SB203580 binds both isoforms. Thus, it is possible that the effect observed with SB203580 on Band 3 phosphorylation was mediated via p38 MAPKβ. In addition, our observations of p38 MAPK-mediated oxidative stress were limited to diamide treatments. As we have not tested other oxidant agents, oxidative injury may occur without p38 MAPK activation.
5. Conclusion
This study supports previous work that demonstrated p38-MAPK-mediated eryptosis and hemolysis under different extracellular stress stimuli, such as hyperosmotic shock, antimicrobial and antifungal agents, chemotherapy drugs, and metal pollutants [27-33]. Our study expands on these finding by identifying kinases associated with p38 MAPK regulation of hemolysis in response to oxidative and osmotic stressors, and in hemoglobinopathies. Further characterization of these signaling pathways could lead to new MAPK-based therapeutic approaches to mitigate erythrocyte damage and hemolysis in hemoglobinopathies.
Supplementary Material
Acknowledgments
This study was supported by the National Heart, Lung, and Blood Institute (NHLBI) grant number R01 HL134653 (T.K). The corresponding author (T.K) wishes to acknowledge Dr. Mark Gladwin from the University of Pittsburgh for his scientific advice and mentorship support with this study.
Footnotes
Declaration of Competing Interest
Though unrelated to the contents of the manuscript, T.K is a coinventor of a nanoparticle-based technology for blood preservation through collaboration with XHEME Inc. All the other authors disclose no conflicts of interest relevant to this study.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cellsig.2022.110450.
Data availability
Data will be made available on request.
References
- [1].Roux PP, Blenis J, ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions, Microbiol. Mol. Biol. Rev 68 (2004) 320–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Zhou X, Naguro I, Ichijo H, Watanabe K, Mitogen-activated protein kinases as key players in osmotic stress signaling, Biochim. Biophys. Acta 1860 (2016) 2037–2052. [DOI] [PubMed] [Google Scholar]
- [3].Hattori K, Ishikawa H, Sakauchi C, Takayanagi S, Naguro I, Ichijo H, Cold stress-induced ferroptosis involves the ASK1-p38 pathway, EMBO Rep. 18 (2017) 2067–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Gong X, Liu A, Ming X, Deng P, Jiang Y, UV-induced interaction between p38 MAPK and p53 serves as a molecular switch in determining cell fate, FEBS Lett. 584 (2010) 4711–4716. [DOI] [PubMed] [Google Scholar]
- [5].Son Y, Cheong YK, Kim NH, Chung HT, Kang DG, Pae HO, Mitogen-activated protein kinases and reactive oxygen species: how can ROS activate MAPK pathways? J. Signal Transduct 2011 (2011), 792639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Cuenda A, Rousseau S, p38 MAP-kinases pathway regulation, function and role in human diseases, Biochim. Biophys. Acta 1773 (2007) 1358–1375. [DOI] [PubMed] [Google Scholar]
- [7].Gupta J, Nebreda AR, Roles of p38 alpha mitogen-activated protein kinase in mouse models of inflammatory diseases and cancer, FEBS J. 282 (2015) 1841–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Naor Z, Benard O, Seger R, Activation of MAPK cascades by G-protein-coupled receptors: the case of gonadotropin-releasing hormone receptor, Trends Endocrinol. Metab 11 (2000) 91–99. [DOI] [PubMed] [Google Scholar]
- [9].Remy G, Risco AM, Inesta-Vaquera FA, Gonzalez-Teran B, Sabio G, Davis RJ, Cuenda A, Differential activation of p38MAPK isoforms by MKK6 and MKK3, Cell. Signal 22 (2010) 660–667. [DOI] [PubMed] [Google Scholar]
- [10].Plotnikov A, Zehorai E, Procaccia S, Seger R, The MAPK cascades: signaling components, nuclear roles and mechanisms of nuclear translocation, Biochim. Biophys. Acta 1813 (2011) 1619–1633. [DOI] [PubMed] [Google Scholar]
- [11].Germann UA, Alam JJ, P38alpha MAPK signaling-a robust therapeutic target for Rab5-mediated neurodegenerative disease, Int. J. Mol. Sci 21 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Xing L, Clinical candidates of small molecule p38 MAPK inhibitors for inflammatory diseases, MAP Kinase 4 (2016). [Google Scholar]
- [13].Genovese MC, Cohen SB, Wofsy D, Weinblatt ME, Firestein GS, Brahn E, Strand V, Baker DG, Tong SE, A 24-week, randomized, double-blind, placebo-controlled, parallel group study of the efficacy of oral SCIO-469, a p38 mitogen-activated protein kinase inhibitor, in patients with active rheumatoid arthritis, J. Rheumatol 38 (2011) 846–854. [DOI] [PubMed] [Google Scholar]
- [14].Navas TA, Nguyen AN, Hideshima T, Reddy M, Ma JY, Haghnazari E, Henson M, Stebbins EG, Kerr I, O’Young G, Kapoun AM, Chakravarty S, Mavunkel B, Perumattam J, Luedtke G, Dugar S, Medicherla S, Protter AA, Schreiner GF, Anderson KC, Higgins LS, Inhibition of p38alpha MAPK enhances proteasome inhibitor-induced apoptosis of myeloma cells by modulating Hsp27, Bcl-X(L), Mcl-1 and p53 levels in vitro and inhibits tumor growth in vivo, Leukemia 20 (2006) 1017–1027. [DOI] [PubMed] [Google Scholar]
- [15].Grimes JM, Grimes KV, p38 MAPK inhibition: a promising therapeutic approach for COVID-19, J. Mol. Cell. Cardiol 144 (2020) 63–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Laufer S, Lehmann F, Investigations of SCIO-469-like compounds for the inhibition of p38 MAP kinase, Bioorg. Med. Chem. Lett 19 (2009) 1461–1464. [DOI] [PubMed] [Google Scholar]
- [17].Schultze SM, Mairhofer A, Li D, Cen J, Beug H, Wagner EF, Hui L, p38alpha controls erythroblast enucleation and Rb signaling in stress erythropoiesis, Cell Res. 22 (2012) 539–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hu P, Nebreda AR, Hanenberg H, Kinnebrew GH, Ivan M, Yoder MC, Filippi MD, Broxmeyer HE, Kapur R, P38alpha/JNK signaling restrains erythropoiesis by suppressing Ezh2-mediated epigenetic silencing of Bim, Nat. Commun 9 (2018) 3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Tamura K, Sudo T, Senftleben U, Dadak AM, Johnson R, Karin M, Requirement for p38alpha in erythropoietin expression: a role for stress kinases in erythropoiesis, Cell 102 (2000) 221–231. [DOI] [PubMed] [Google Scholar]
- [20].Dalmas DA, Tierney LA, Zhang C, Narayanan PK, Boyce RW, Schwartz LW, Frazier KS, Scicchitano MS, Effects of p38 MAP kinase inhibitors on the differentiation and maturation of erythroid progenitors, Toxicol. Pathol 36 (2008) 958–971. [DOI] [PubMed] [Google Scholar]
- [21].Geest CR, Coffer PJ, MAPK signaling pathways in the regulation of hematopoiesis, J. Leukoc. Biol 86 (2009) 237–250. [DOI] [PubMed] [Google Scholar]
- [22].Risco A, Cuenda A, New insights into the p38gamma and p38delta MAPK pathways, J. Signal Transduct 2012 (2012), 520289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Uddin S, Ah-Kang J, Ulaszek J, Mahmud D, Wickrema A, Differentiation stage-specific activation of p38 mitogen-activated protein kinase isoforms in primary human erythroid cells, Proc. Natl. Acad. Sci. U. S. A 101 (2004) 147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Nagata Y, Takahashi N, Davis RJ, Todokoro K, Activation of p38 MAP kinase and JNK but not ERK is required for erythropoietin-induced erythroid differentiation, Blood 92 (1998) 1859–1869. [PubMed] [Google Scholar]
- [25].Kapur R, Chandra S, Cooper R, McCarthy J, Williams DA, Role of p38 and ERK MAP kinase in proliferation of erythroid progenitors in response to stimulation by soluble and membrane isoforms of stem cell factor, Blood 100 (2002) 1287–1293. [PubMed] [Google Scholar]
- [26].Pace BS, Qian XH, Sangerman J, Ofori-Acquah SF, Baliga BS, Han J, Critz SD, p38 MAP kinase activation mediates gamma-globin gene induction in erythroid progenitors, Exp. Hematol 31 (2003) 1089–1096. [DOI] [PubMed] [Google Scholar]
- [27].Gatidis S, Zelenak C, Fajol A, Lang E, Jilani K, Michael D, Qadri SM, Lang F, p38 MAPK activation and function following osmotic shock of erythrocytes, Cell. Physiol. Biochem 28 (2011) 1279–1286. [DOI] [PubMed] [Google Scholar]
- [28].Peter T, Bissinger R, Liu G, Lang F, Anidulafungin-induced suicidal erythrocyte death, Cell. Physiol. Biochem 38 (2016) 2272–2284. [DOI] [PubMed] [Google Scholar]
- [29].Egler J, Zierle J, Lang F, Stimulating effect of manumycin a on suicidal erythrocyte death, Cell. Physiol. Biochem 38 (2016) 1147–1156. [DOI] [PubMed] [Google Scholar]
- [30].Alfhili MA, Weidner DA, Lee MH, Disruption of erythrocyte membrane asymmetry by triclosan is preceded by calcium dysregulation and p38 MAPK and RIP1 stimulation, Chemosphere 229 (2019) 103–111. [DOI] [PubMed] [Google Scholar]
- [31].Alfhili MA, Basudan AM, Aljaser FS, Dera A, Alsughayyir J, Bioymifi, a novel mimetic of TNF-related apoptosis-induced ligand (TRAIL), stimulates eryptosis, Med. Oncol 38 (2021) 138. [DOI] [PubMed] [Google Scholar]
- [32].Sultan SA, Khawaji MH, Alsughayyir J, Alfhili MA, Alamri HS, Alrfaei BM, Antileukemic activity of sulfoxide nutraceutical allicin against THP-1 cells is associated with premature phosphatidylserine exposure in human erythrocytes, Saudi J. Biol. Sci 27 (2020) 3376–3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Alfhili MA, Alamri HS, Alsughayyir J, Basudan AM, Induction of hemolysis and eryptosis by occupational pollutant nickel chloride is mediated through calcium influx and p38 MAP kinase signaling, Int. J. Occup. Med. Environ. Health 35 (2022) 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Zennadi R, Whalen EJ, Soderblom EJ, Alexander SC, Thompson JW, Dubois LG, Moseley MA, Telen MJ, Erythrocyte plasma membrane-bound ERK1/2 activation promotes ICAM-4-mediated sickle red cell adhesion to endothelium, Blood 119 (2012) 1217–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Manci EA, Hillery CA, Bodian CA, Zhang ZG, Lutty GA, Coller BS, Pathology of Berkeley sickle cell mice: similarities and differences with human sickle cell disease, Blood 107 (2006) 1651–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ryan TM, Ciavatta DJ, Townes TM, Knockout-transgenic mouse model of sickle cell disease, Science 278 (1997) 873–876. [DOI] [PubMed] [Google Scholar]
- [37].Yang B, Kirby S, Lewis J, Detloff PJ, Maeda N, Smithies O, A mouse model for beta 0-thalassemia, Proc. Natl. Acad. Sci. U. S. A 92 (1995) 11608–11612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Davidson W, Frego L, Peet GW, Kroe RR, Labadia ME, Lukas SM, Snow RJ, Jakes S, Grygon CA, Pargellis C, Werneburg BG, Discovery and characterization of a substrate selective p38alpha inhibitor, Biochemistry 43 (2004) 11658–11671. [DOI] [PubMed] [Google Scholar]
- [39].Zwart A, van Assendelft OW, Bull BS, England JM, Lewis SM, Zijlstra WG, Recommendations for reference method for haemoglobinometry in human blood (ICSH standard 1995) and specifications for international haemiglobinocyanide standard (4th edition), J. Clin. Pathol 49 (1996) 271–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Bucx MJL, Breed WPM, Hoffmann JJML, Comparison of acidified glycerol lysis test, pink test and osmotic fragility test in hereditary spherocytosis: effect of incubation, Eur. J. Haematol 40 (1988) 227–231. [DOI] [PubMed] [Google Scholar]
- [41].Kanias T, Sinchar D, Osei-Hwedieh D, Baust JJ, Jordan A, Zimring JC, Waterman HR, de Wolski KS, Acker JP, Gladwin MT, Testosterone-dependent sex differences in red blood cell hemolysis in storage, stress, and disease, Transfusion 56 (2016) 2571–2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Pantaleo A, Ferru E, Carta F, Mannu F, Simula LF, Khadjavi A, Pippia P, Turrini F, Irreversible AE1 tyrosine phosphorylation leads to membrane vesiculation in G6PD deficient red cells, PLoS One 6 (2011), e15847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Pantaleo A, Ferru E, Giribaldi G, Mannu F, Carta F, Matte A, de Franceschi L, Turrini F, Oxidized and poorly glycosylated band 3 is selectively phosphorylated by Syk kinase to form large membrane clusters in normal and G6PD-deficient red blood cells, Biochem. J 418 (2009) 359–367. [DOI] [PubMed] [Google Scholar]
- [44].Dodge JT, Mitchell C, Hanahan DJ, The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes, Arch. Biochem. Biophys 100 (1963) 119–130. [DOI] [PubMed] [Google Scholar]
- [45].Carriere A, Ray H, Blenis J, Roux PP, The RSK factors of activating the Ras/MAPK signaling cascade, Front. Biosci 13 (2008) 4258–4275. [DOI] [PubMed] [Google Scholar]
- [46].Reustle A, Torzewski M, Role of p38 MAPK in atherosclerosis and aortic valve sclerosis, Int. J. Mol. Sci 19 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Noomuna P, Risinger M, Zhou S, Seu K, Man Y, An R, Sheik DA, Wan J, Little JA, Gurkan UA, Turrini FM, Kalfa T, Low PS, Inhibition of Band 3 tyrosine phosphorylation: a new mechanism for treatment of sickle cell disease, Br. J. Haematol 190 (2020) 599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Morgan D, Berggren KL, Spiess CD, Smith HM, Tejwani A, Weir SJ, Lominska CE, Thomas SM, Gan GN, Mitogen-activated protein kinase-activated protein kinase-2 (MK2) and its role in cell survival, inflammatory signaling, and migration in promoting cancer, Mol. Carcinog 61 (2022) 173–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Di Stazio M, Bigoni S, Iuso N, Vuch J, Selvatici R, Ulivi S, d’Adamo PA, Identification of a new mutation in RSK2, the gene for Coffin-Lowry syndrome (CLS), in two related patients with mild and atypical phenotypes, Brain Sci. 11 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Kang S, Chen J, Targeting RSK2 in human malignancies, Expert Opin. Ther. Targets 15 (2011) 11–20. [DOI] [PubMed] [Google Scholar]
- [51].Page GP, Kanias T, Guo YJ, Lanteri MC, Zhang X, Mast AE, Cable RG, Spencer BR, Kiss JE, Fang F, Endres-Dighe SM, Brambilla D, Nouraie M, Gordeuk VR, Kleinman S, Busch MP, Gladwin MT, National Heart L, Blood Institute Recipient Epidemiology Donor Evaluation Study IIIp, Multiple-ancestry genome-wide association study identifies 27 loci associated with measures of hemolysis following blood storage, J. Clin. Invest 131 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Moens U, Kostenko S, Structure and function of MK5/PRAK: the loner among the mitogen-activated protein kinase-activated protein kinases, Biol. Chem 394 (2013) 1115–1132. [DOI] [PubMed] [Google Scholar]
- [53].Coulombe P, Meloche S, Atypical mitogen-activated protein kinases: structure, regulation and functions, Biochim. Biophys. Acta 1773 (2007) 1376–1387. [DOI] [PubMed] [Google Scholar]
- [54].Kanias T, Lanteri MC, Page GP, Guo Y, Endres SM, Stone M, Keating S, Mast AE, Cable RG, Triulzi DJ, Kiss JE, Murphy EL, Kleinman S, Busch MP, Gladwin MT, Ethnicity, sex, and age are determinants of red blood cell storage and stress hemolysis: results of the REDS-III RBC-Omics study, Blood Adv. 1 (2017) 1132–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Huber S, Schrader J, Fritz G, Presser K, Schmitt S, Waisman A, Luth S, Blessing M, Herkel J, Schramm C, P38 MAP kinase signaling is required for the conversion of CD4+CD25− T cells into iTreg, PLoS One 3 (2008), e3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].He T, Liu S, Chen S, Ye J, Wu X, Bian Z, Chen X, The p38 MAPK inhibitor SB203580 abrogates tumor necrosis factor-induced proliferative expansion of mouse CD4(+)Foxp3(+) regulatory T cells, Front. Immunol 9 (2018) 1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Li WH, Zhang L, Lyte P, Rodriguez K, Cavender D, Southall MD, p38 MAP kinase inhibition reduces Propionibacterium acnes-induced inflammation in vitro, Dermatol Ther. (Heidelb.) 5 (2015) 53–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.