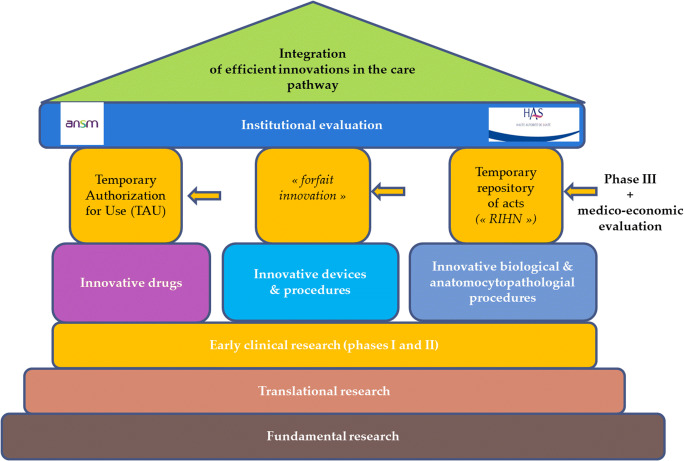
Fig. 1.
Reimbursement decision-making processa for innovations in France. Source: Adapted from the Instruction DGOS/PF4 no 2015–258 of the 31st July of the Ministry of Health (http://solidarites-sante.gouv.fr/fichiers/bo/2015/15-08/ste_20150008_0000_0113.pdf). aBefore considering a possible coverage by the insurance system, evidence of their validity in terms of reliability, accuracy, reproducibility, and performance should be demonstrated. After examination, these procedures can be listed in a temporary repository of innovative acts. This registration is only possible if a prospective and comparative collection of data to validate the clinical effectiveness, the efficiency of these procedures, is carried out. After 3 years, if data are considered robust, a reimbursement decision by the collectivity will be or not taken. A similar system exists for innovative drugs and medical devices