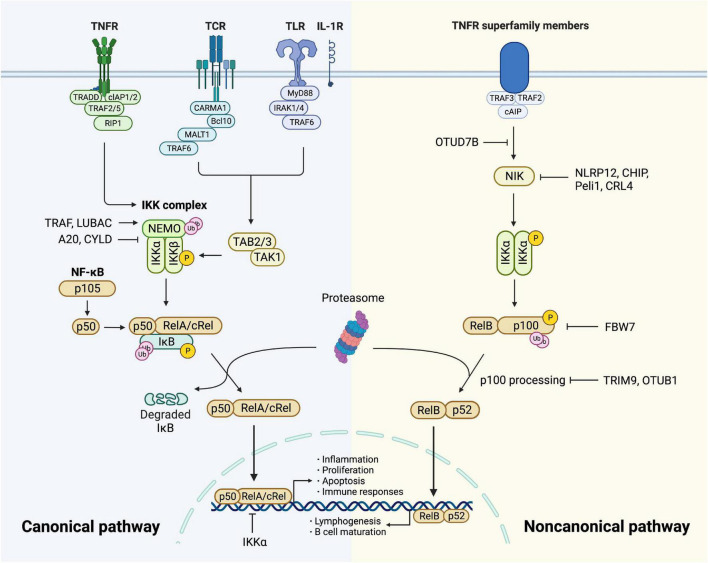
FIGURE 1.
Canonical and non-canonical NF-κB pathway. The canonical pathway is induced via activation of receptors like TNFR, TCR, TLR, and IL-1R. When TNFR is activated by ligands, it recruits TRADD and drives the assembly of cIAP, TRAF, and RIP1 which is then recruited to NEMO and subsequent formation of IKK complex. TCR recruits CBM complex which is then ubiquitinated by TRAF6, resulting in the activation of TAK1. TLR and IL-1R recruits MyD88 and IRAK1/4, followed by TRAF6 to activate TAK and then IKK complex. TAK1 phosphorylates and activates IKK complex via phosphorylation of IKKβ. Then IκB family members phosphorylated by IKK undergo ubiquitin-dependent degradation, resulting in the release of NF-κB dimers. The canonical NF-κB pathway is regulated precisely. IKKα impedes RelA binding to DNA in nucleus. A20 and CYLD destabilize IKK complex via their deubiquitination activities. The activity of NF-κB is increased by TRAF- and LUBAC-mediated ubiquitination of NEMO. The non-canonical NF-κB pathway is initiated from the stimulation of specific TNFRs, which triggers the recruitment of TRAF3-TRAF2-cIAP and eventually results in stabilization and accumulation of NIK, which is impeded by deubiquitinase OTUD7B. Degradation of NIK is promoted by NLRP12, CHIP, Peli1, and CRL4. NIK phosphorylates and activates IKKα, triggering phosphorylation and ubiquitylation of p100. RelB and p52 generated from p100 constitute NF-κB heterodimer that conducts nuclear translocation and gene transcription. TRIM9 and OTUB1 inhibit p100 processing and FBW7 mediates p100 destruction.