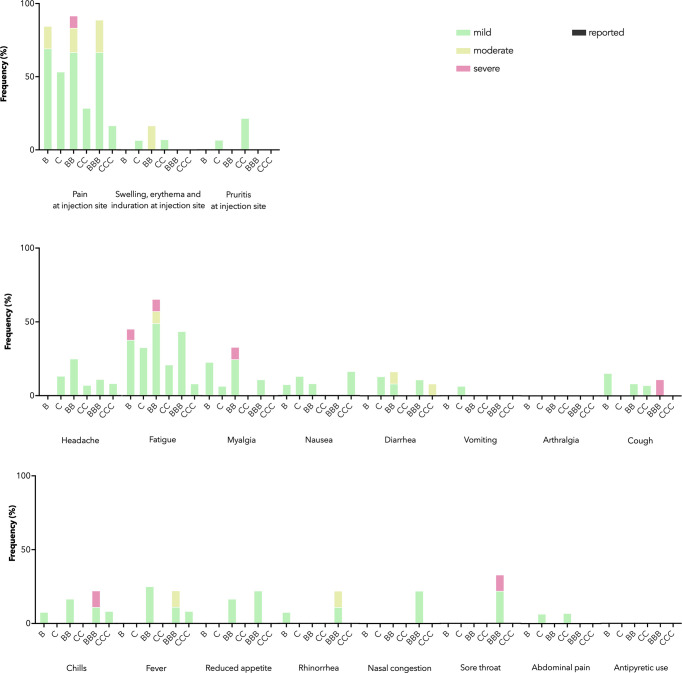
Figure 1.
Adverse reactions (ARs) and antipyretic use reported 7 days after each dose by vaccine brand. B, BB, and BBB refer to 1, 2, and 3 doses of BNT162b2 while C, CC, and CCC refer to 1, 2, and 3 doses of CoronaVac. Stacked bar chart shows ARs by maximal severity in different colors. Severity was self-graded by participants, according to whether the AR affected daily activity (mild – tolerable, not affecting daily activities, moderate - performance of some daily activities affected, or severe - performance of some daily activities prevented), or for swelling, erythema and induration at injection site, the diameter affected (mild – 2-5 cm, moderate – 5-10 cm, severe – above 10 cm), or for fever, the body temperature (mild – 38.0-38.4°C, moderate – 38.5-38.9°C, severe – 39.0 °C or above).