Abstract
Backgrounds
Malnutrition and systemic inflammatory responses are associated with poor overall survival (OS) in lung cancer patients, but it remains unclear which biomarkers are better for predicting their prognosis. This study tried to determine the best one among the existing common nutrition/inflammation‐based indicators of OS for patients with lung cancer.
Materials and methods
There were 16 nutrition or systemic inflammation‐based indicators included in this study. The cut‐off points for the indicators were calculated using maximally selected rank statistics. The OS was evaluated using the Kaplan–Meier estimator, and univariate and multivariate Cox proportional hazard models were used to determine the relationship between the indicators and OS. A time‐dependent receiver operating characteristic curves (time‐ROC) and C‐index were calculated to assess the predictive ability of the different indicators.
Results
There were 1772 patients with lung cancer included in this study. In univariate analysis, all 16 indicators were significantly associated with OS of the patients (all P < 0.001). Except for platelet‐to‐lymphocyte ratio, all other indicators were independent predictors of OS in multivariate analysis (all P < 0.05). Low advanced lung cancer inflammation index (ALI) was associated with higher mortality risk of lung cancer [hazard ratio, 1.30; 95% confidence interval (CI), 1.13–1.49]. The results of the time‐AUC and C‐index analyses indicated that the ALI (C‐index: 0.611) had the best predictive ability on the OS in patients with lung cancer. In different sub‐groups, the ALI was the best indicator for predicting the OS of lung cancer patients regardless of sex (C‐index, 0.609 for men and 0.613 for women) or smoking status (C‐index, 0.629 for non‐smoker and 0.601 for smoker) and in patients aged <65 years (C‐index, 0.613). However, the modified Glasgow prognostic score was superior to the other indicators in non‐small cell lung cancer patients (C‐index, 0.639) or patients aged ≥65 years (C‐index, 0.610), and the glucose‐to‐lymphocyte ratio performed better prognostic ability in patients with small cell lung cancer (C‐index, 0.601).
Conclusions
The prognostic ability of the ALI is superior to the other inflammation/nutrition‐based indicators for all patients with lung cancer.
Keywords: Lung cancer, Prognosis, Inflammation indicators
Introduction
Lung cancer is one of the most commonly diagnosed cancers and the leading cause of mortality worldwide, accounting for approximately 18% of all cancer deaths. 1 Despite major advances in diagnostic strategies and effective new treatment modalities (such as immunotherapy), the 5‐year survival rate of lung cancer is only 10–20%. 2 Predicting the prognosis of patients with lung cancer is challenging; therefore, there is an urgent need for effective biomarkers for predicting patient survival to help identify patients and conduct timely and effective treatment. The American Joint Committee on Cancer tumour node metastasis (TNM) classification and staging system has proven to be predictive of overall survival (OS) in patients with lung cancer, and a number of clinical indicators 3 such as smoking habit, gender, age, weight loss, 4 performance status, 5 and some inflammatory indicators 6 seem to be associated with the prognosis of lung cancer patients.
Recently, elevating evidences have shown that multiple nutrition/inflammation‐related factors can be used as effective prognostic predictors in lung cancer. The markers of the systemic inflammatory response, such as the plasma C‐reactive protein (CRP) levels 7 , platelet‐to‐lymphocyte ratio (PLR), 6 and the neutrophil‐to‐lymphocyte ratio (NLR) 8 have been shown to play an important role in the progression and prognosis of patients with lung cancer. The prognostic significance of the advanced lung cancer inflammation index (ALI), which is calculated by multiplying the body mass index (BMI) by serum albumin/NLR, has been described in several studies that showed that low ALI is associated with an unfavourable prognosis in lung cancer. 9 , 10 , 11 A high systemic immune‐inflammation index (SII) 12 and CRP/albumin ratio (CAR) 13 are also biomarkers that predict poor prognosis in lung cancer. A low albumin‐to‐globulin ratio (AGR) is an independent risk factor for poor OS in non‐small cell lung cancer (NSCLC) and small‐cell lung cancer (SCLC). 14 The Glasgow prognostic score (GPS) and modified Glasgow prognostic score (mGPS), which consist of albumin and CRP levels, can also predict the OS of patients with lung cancer. 15 Moreover, nutrition‐based indicators such as the geriatric nutritional risk index (GNRI), 16 nutritional risk index (NRI), 17 and prognostic nutritional index (PNI) 18 have been shown to be prognostic factors for predicting outcomes in patients with lung cancer. In addition, the controlling nutritional status (CONUT) score is a novel prognostic parameter for various cancers, including lung cancer. 19 The baseline glucose‐to‐lymphocyte ratio (GLR) is an independent prognostic factor for patients with pancreatic cancer 20 and gallbladder cancer. 21 The lymphocyte‐to‐CRP ratio (LCR), lymphocyte CRP score (LCS), 22 , 23 and modified GNRI (mGNRI) are useful prognostic biomarkers for several types of cancer in recent studies. 24 However, the prognostic significance of the GLR, mGNRI, and LCS in lung cancer remains unknown.
Although studies have confirmed that some of the aforementioned nutrition/inflammation‐related indicators have value for predicting the survival of lung cancer patients, it is necessary to confirm which indicators are the best prognosis predictors for lung cancer patients. In this study, we evaluated and compared the predictive and the prognostic roles of 16 biomarkers of malnutrition/inflammation‐based indicators on the OS of patients with lung cancer. We also assessed which indicator was the unique among different sub‐groups.
Material and methods
Participants
All patients were recruited from the Investigation on Nutrition Status and its Clinical Outcome of Common Cancers (INSCOC) project, which recruited participants from multiple clinical centres across China from 2013. The trial is registered at http://www.chictr.org.cn under the registration number ChiCTR1800020329. Data were prospectively collected from multiple centres across China. The design, methods and development of the INSCOC study were performed as described previously. 25 All patients who had missing records for age, height, serum globulin levels, fasting blood glucose data, neutrophil counts, platelet counts, serum albumin levels, CRP levels, cholesterol concentration, or lymphocyte counts were excluded from the study. Patients without lung cancer were also excluded from the study. A total of 1772 patients with lung cancer were selected for this study. A flow chart of the participant selection is shown in Figure 1. All selected patients signed informed consent forms prior to the study initiation within 48 h of hospital admission. Patients who could not communicate and/or were unable to provide verbal consent were excluded from the study. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the institutional review committee of Beijing Shijitan Hospital.
Figure 1.
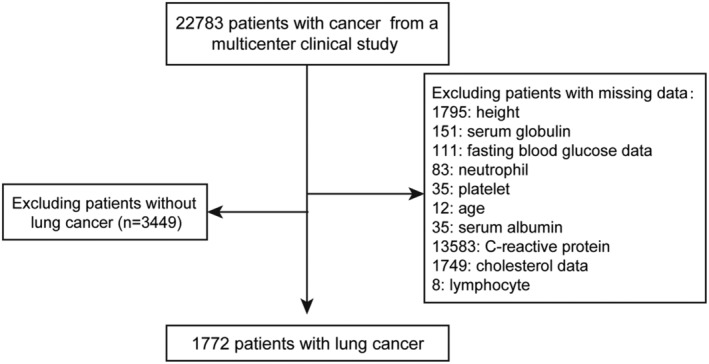
The flow chart.
Baseline data collection
The baseline information of the patients included age, sex, complications, smoking status, the family history of tumours, the pathological type of lung cancer, and alcohol consumption. A dietitian or clinician conducted a comprehensive interview with each patient to acquire recent preoperative nutritional information, including the Nutritional Risk Screening 2002 score, Patient‐Generated Subjective Global Assessment (PG‐SGA) score, Karnofsky Performance Score (KPS), and anthropometric measurements including height, body weight, and handgrip strength. The tumour stage of solid tumours was evaluated based on the 8th edition of the American Joint Committee on Cancer TNM staging system.
Measurements of nutrition/inflammation‐based indicator in routine blood tests
Routine blood examinations were obtained after at least 9 h of fasting, within 24 h of hospitalization, and included the levels of albumin, glucose, globulin, haemoglobin, C‐reaction protein (CRP) and total cholesterol, and lymphocyte, neutrophil, and platelet counts. The 16 nutrition/inflammation‐based indicators used in this study included the ALI, SII, PLR, NLR, PNI, GLR, LCR AGR, GNRI, mGNRI, NRI, mGPS, LCS, and CONUT score. The definition of the LCS, CONUT scores, and mGPS were determined based on previous reports. The calculation methods for the combination of each nutrition/inflammation‐based indicator are shown in Table S1.
Statistical analysis
All statistical analyses were performed using SPSS Version 21 software (SPSS, IBM, Armonk, NY, USA) and R Version 4.0.2, including the R packages ‘survminer’, ‘survival’, ‘rms’, ‘ggplot2’, ‘forestplot’, ‘timeROC’, and ‘maxstat’. Variables are expressed as the mean ± standard deviation, as median (interquartile range), or in absolute number and proportion as appropriate (continuous or categorical variables). The differences between the groups were evaluated using the Mann–Whitney test (for continuous variables that were not normally distributed), and the chi‐square test (for categorical variables). We dichotomized the continuous nutrition/inflammation‐based indicators based on the optimal cut‐off points calculated using maximally selected rank statistics. The OS was evaluated using Kaplan–Meier curves and analysed using the two‐sided log‐rank test. The Cox proportional hazard model was used to assess the relationship between the nutrition/inflammation‐based indicators and OS in patients with lung cancer. The predictive accuracy of each indicator was assessed using the C‐index and time‐dependent receiver operating characteristic curves (time‐ROC). To determine whether the same indicator was applicable across the sub‐groups, and to gain insight into the most useful biomarker in the different sub‐groups, we carried out a sub‐group analysis of age, sex, lung cancer types, and smoking status in patients with lung cancer. Two‐sided P < 0.05 was considered statistical significance.
Results
Characteristics of the patients
A total of 1772 patients were included in the study. Of these, 791 (44.6%) had NSCLC, and 233 (13.1%) had SCLC; 748 (42.2%) patients with lung cancer did not have a clear pathological classification. The patient demographics are shown in Table 1. In our population, the mean age was 60.78 ± 9.46 years, and males were prevalent (n = 1134, 64.0%). Of all the patients, 60.7% were smokers. The prevalence of distant metastasis was high (51.7%). Of all patients in this study, 403 (22.7%) underwent surgery, and most patients were treated with chemotherapy (70.4%). Furthermore, the patients enrolled in the study had a poor nutritional status with a high PG‐SGA score (5.27 ± 4.43).
Table 1.
Baseline clinico‐pathological characteristics of patients with lung cancer
| All (n = 1772) | |
|---|---|
| Age, mean ± SD | 60.78 ± 9.46 |
| Sex, n (%) | |
| Men | 1134 (64.0) |
| Women | 638 (36.0) |
| BMI, mean ± SD | 22.89 ± 3.40 |
| Pathological type, n (%) | |
| NSCLC | 791 (44.6) |
| SCLC | 233 (13.1) |
| Other | 748 (42.2) |
| Smoke, n (%) | |
| No | 696 (39.3) |
| Yes | 1076 (60.7) |
| Alcohol, n (%) | |
| No | 1314 (74.2) |
| Yes | 458 (25.8) |
| Co‐morbidities, n (%) | |
| No | 1062 (59.9) |
| Yes | 710 (40.1) |
| Tumour history, n (%) | |
| No | 1456 (82.2) |
| Yes | 316 (17.8) |
| TNM stage, n (%) | |
| I | 129 (7.3) |
| II | 264 (14.9) |
| III | 462 (26.1) |
| IV | 917 (51.7) |
| Surgery, n (%) | |
| No | 1369 (77.3) |
| Yes | 403 (22.7) |
| Radiotherapy, n (%) | |
| No | 1582 (89.3) |
| Yes | 190 (10.7) |
| Chemotherapy, n (%) | |
| No | 525 (29.6) |
| Yes | 1247 (70.4) |
| KPS, mean ± SD | 85.91 ± 11.75 |
| <70 | 93 (5.2) |
| ≥70 | 1679 (94.8) |
| PG‐SGA, mean ± SD | 5.27 ± 4.43 |
| Total protein, median (IQR) | 68.95 (64.93–73) |
| Albumin, median (IQR) | 39.3 (36.28–42.1) |
| C‐reaction protein, median (IQR) | 5.595 (3.02–20.925) |
| Glucose, median (IQR) | 5.39 (4.91–6.0925) |
| Haemoglobin, median (IQR) | 131.0 (117.0–142.0) |
| Neutrophil, median (IQR) | 4.165 (2.97–5.74) |
| Lymphocyte, median (IQR) | 1.57 (1.18–2.01) |
| Red blood cell, median (IQR) | 4.35 (3.94–4.72) |
| Platelets, median (IQR) | 238 (187–296) |
| NLR, median (IQR) | 2.61 (1.78–4.12) |
| PLR, median (IQR) | 151.42 (108.62–211.65) |
| GLR, median (IQR) | 3.50 (2.64–4.94) |
| ALI, median (IQR) | 34.84 (20.31–53.62) |
| SII, median (IQR) | 617.94 (365.14–1040.58) |
| CAR, median (IQR) | 0.14 (0.07–0.55) |
| CONUT, n (%) | |
| <2 | 852 (48.1) |
| ≥2 | 920 (51.9) |
| mGPS, n (%) | |
| 0 | 1099 (62.0) |
| 1 | 453 (25.6) |
| 2 | 220 (12.4) |
| GNRI, median (IQR) | 98.88 (93.22–103.20) |
| mGNRI, median (IQR) | 43.25 (41.61–46.46) |
| AGR, median (IQR) | 1.34 (1.15–1.54) |
| PNI, median (IQR) | 47.4 (43.5–51.4) |
| NRI, median (IQR) | 100.06 (94.26–104.50) |
| LCS, n (%) | |
| 0 | 358 (20.2) |
| 1 | 1209 (68.2) |
| 2 | 205 (11.6) |
| LCR, median (IQR) | 2694.61 (676.41–6432.80) |
AGR, albumin‐to‐globulin ratio; ALI, advanced lung cancer inflammation index; BMI, body mass index; CAR, C‐reactive protein‐to‐albumin ratio; CONUT score, controlling nutritional status score; GLR, glucose‐to‐lymphocyte ratio; GNRI, geriatric nutritional risk index; KPS, Karnofsky performance scoring; LCR, lymphocyte‐to‐C‐reactive protein ratio; mGNRI, modified geriatric nutritional risk index; mGPS, modified Glasgow prognostic score; NLR, neutrophil‐to‐lymphocyte ratio; NRI, nutritional risk index; LCS, lymphocyte‐to‐C‐reactive protein ratio score; NSCLC, non‐small cell lung cancer; PG‐SGA, Patient‐Generated Subjective Global Assessment; PLR, platelet‐to‐lymphocyte ratio; PNI, prognostic nutritional index; SCLC, small cell lung cancer; SD, standard deviation; SII, systemic immune‐inflammation index.
Association of inflammation/nutrition‐based indicators and OS in lung cancer patients
After nearly 8 years of follow‐up, 926 of the lung cancer patients had died; the median OS time was 26.1 months. In particular, the median OS times for SCLC and NSCLC were 22.2 months and 32.5 months, respectively. The cut‐off points of 16 inflammation and malnutrition‐based indicators were 3.36 (NLR), 206.96 (PLR), 5.27 (GLR), 705.03 (SII), 34.19 (ALI), 0.18 (CAR), 96.05 (GNRI), 43.83 (mGNRI), 1.24 (AGR), 48.60 (PNI), 97.17 (NRI), 2728.94 (LCR), and 9.02 (CRP). The non‐linear correlation between these nutrition/inflammation‐based indicators and the mortality of patients with lung cancer is shown in Figure S1. Univariate and multivariate analyses showed that the ALI, SII, NLR, PNI, GLR, CRP, LCR, LCS, CAR, AGR, GNRI, mGNRI, NRI, mGPS, and CONUT score, but not PLR, were independent risk factors for elevated mortality in patients with lung cancer (Table 2). Kaplan–Meier curves showed that lung cancer patients with malnutrition and inflammation had a more unfavourable OS than those without malnutrition or inflammation (Figures 2 and S2).
Table 2.
The univariate and multivariate cox analysis of fifteen indicators in patients with lung cancer
| Model0 | Model1 | Model2 | |||||
|---|---|---|---|---|---|---|---|
| Cases/controls | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| NLR | |||||||
| Per SD (increased) | 1.17 (1.12,1.22) | <0.001 | 1.09 (1.03,1.15) | 0.002 | 1.05 (0.99,1.11) | 0.102 | |
| <3.36 | 527/634 | Reference | Reference | Reference | |||
| ≥3.36 | 399/212 | 1.85 (1.62,2.10) | <0.001 | 1.42 (1.24,1.62) | <0.001 | 1.29 (1.13,1.48) | <0.001 |
| PLR | |||||||
| Per SD (increased) | 1.10 (1.04,1.17) | 0.001 | 1.04 (0.97,1.11) | 0.253 | 0.99 (0.93,1.06) | 0.786 | |
| <207.00 | 644/665 | Reference | Reference | Reference | |||
| ≥207.00 | 282/181 | 1.40 (1.22,1.62) | <0.001 | 1.17 (1.02,1.35) | 0.028 | 1.06 (0.92,1.23) | 0.403 |
| GLR | |||||||
| Per SD (increased) | 1.15 (1.09,1.21) | <0.001 | 1.09 (1.04,1.16) | 0.001 | 1.06 (1.00,1.12) | 0.047 | |
| <5.27 | 668/710 | Reference | Reference | Reference | |||
| ≥5.27 | 258/136 | 1.56 (1.35,1.80) | <0.001 | 1.36 (1.17,1.57) | <0.001 | 1.26 (1.08,1.47) | 0.003 |
| ALI | |||||||
| Per SD (increased) | 0.79 (0.71,0.87) | <0.001 | 0.93 (0.85,1.02) | 0.124 | 0.97 (0.89,1.06) | 0.524 | |
| ≥34.19 | 391/515 | Reference | Reference | Reference | |||
| <34.19 | 535/331 | 1.89 (1.66,2.16) | <0.001 | 1.49 (1.30,1.7) | <0.001 | 1.30 (1.13,1.49) | <0.001 |
| SII | |||||||
| Per SD (increased) | 1.18 (1.12,1.25) | <0.001 | 1.09 (1.02,1.16) | 0.007 | 1.04 (0.98,1.11) | 0.183 | |
| <705.03 | 461/546 | Reference | Reference | Reference | |||
| ≥705.03 | 465/300 | 1.65 (1.45,1.87) | <0.001 | 1.29 (1.13,1.48) | <0.001 | 1.19 (1.04,1.37) | 0.010 |
| CAR | |||||||
| Per SD (increased) | 1.16 (1.09,1.24) | <0.001 | 1.08 (1.01,1.16) | 0.023 | 1.04 (0.97,1.12) | 0.269 | |
| <0.18 | 439/527 | Reference | Reference | Reference | |||
| ≥0.18 | 487/319 | 1.81 (1.59,2.06) | <0.001 | 1.45 (1.27,1.65) | <0.001 | 1.35 (1.18,1.54) | <0.001 |
| GNRI | |||||||
| Per SD (increased) | 0.78 (0.73,0.82) | <0.001 | 0.84 (0.78,0.90) | <0.001 | 0.87 (0.81,0.93) | <0.001 | |
| ≥207.0 | 537/589 | Reference | Reference | Reference | |||
| <207.0 | 389/257 | 1.70 (1.49,1.94) | <0.001 | 1.48 (1.28,1.71) | <0.001 | 1.36 (1.17,1.58) | <0.001 |
| mGNRI | |||||||
| Per SD (increased) | 0.78 (0.71,0.85) | <0.001 | 0.88 (0.81,0.95) | 0.002 | 0.91 (0.84,0.99) | 0.028 | |
| ≥96.05 | 353/437 | Reference | Reference | Reference | |||
| <96.05 | 573/409 | 1.78 (1.55,2.03) | <0.001 | 1.41 (1.22,1.63) | <0.001 | 1.29 (1.12,1.50) | <0.001 |
| AGR | |||||||
| Per SD (increased) | 0.81 (0.76,0.87) | <0.001 | 0.86 (0.80,0.92) | <0.001 | 0.88 (0.82,0.94) | <0.001 | |
| ≥1.24 | 553/584 | Reference | Reference | Reference | |||
| <1.24 | 373/262 | 1.58 (1.39,1.81) | <0.001 | 1.39 (1.21,1.59) | <0.001 | 1.31 (1.14,1.5) | <0.001 |
| PNI | |||||||
| Per SD (increased) | 0.79 (0.74,0.84) | <0.001 | 0.87 (0.81,0.93) | <0.001 | 0.91 (0.85,0.97) | 0.003 | |
| ≥48.60 | 317/408 | Reference | Reference | Reference | |||
| <48.60 | 609/438 | 1.62 (1.42,1.86) | <0.001 | 1.34 (1.17,1.54) | <0.001 | 1.24 (1.08,1.43) | 0.002 |
| NRI | |||||||
| Per SD (increased) | 0.78 (0.73,0.83) | <0.001 | 0.84 (0.78,0.90) | <0.001 | 0.87 (0.81,0.94) | <0.001 | |
| ≥97.17 | 536/589 | Reference | Reference | Reference | |||
| <97.17 | 390/257 | 1.71 (1.5,1.95) | <0.001 | 1.48 (1.28,1.72) | <0.001 | 1.37 (1.18,1.59) | <0.001 |
| LCR | |||||||
| Per SD (increased) | 0.73 (0.64,0.83) | <0.001 | 0.81 (0.72,0.92) | 0.001 | 0.83 (0.74,0.94) | 0.002 | |
| ≥2728.94 | 396/483 | Reference | Reference | Reference | |||
| <2728.94 | 530/363 | 1.77 (1.55,2.02) | <0.001 | 1.41 (1.23,1.61) | <0.001 | 1.28 (1.12,1.47) | <0.001 |
| CRP | |||||||
| Per SD (increased) | 1.18 (1.11,1.26) | <0.001 | 1.10 (1.03,1.18) | 0.006 | 1.05 (0.98,1.13) | 0.146 | |
| <9.02 | 495/567 | Reference | Reference | Reference | |||
| ≥9.02 | 431/279 | 1.76 (1.54,2.00) | <0.001 | 1.38 (1.21,1.58) | <0.001 | 1.27 (1.11,1.46) | <0.001 |
| CONUT | |||||||
| <2 | 388/464 | Reference | Reference | Reference | |||
| ≥2 | 538/382 | 1.49 (1.3,1.69) | <0.001 | 1.27 (1.11,1.46) | <0.001 | 1.20 (1.05,1.38) | 0.007 |
| mGPS | 0.56 (0.49,0.64) | <0.001 | 0.71 (0.62,0.81) | <0.001 | 0.77 (0.67,0.89) | <0.001 | |
| 0 | 518/581 | Reference | Reference | Reference | |||
| 1 | 271/182 | 1.66 (1.43,1.93) | <0.001 | 1.32 (1.13,1.53) | <0.001 | 1.23 (1.05,1.43) | 0.008 |
| 2 | 137/83 | 1.92 (1.59,2.32) | <0.001 | 1.45 (1.19,1.76) | <0.001 | 1.28 (1.04,1.56) | 0.019 |
| P for trend | 1.44 (1.32,1.57) | <0.001 | 1.23 (1.12,1.34) | <0.001 | 1.15 (1.05,1.26) | 0.003 | |
| LCS | |||||||
| 0 | 168/190 | Reference | Reference | Reference | |||
| 1 | 631/578 | 1.47 (1.24,1.74) | <0.001 | 1.2 (1.01,1.43) | 0.037 | 1.15 (0.96,1.37) | 0.121 |
| 2 | 127/78 | 1.96 (1.55,2.47) | <0.001 | 1.44 (1.14,1.81) | 0.002 | 1.23 (0.97,1.56) | 0.092 |
| P for trend | 1.40 (1.25,1.57) | <0.001 | 1.20 (1.07,1.35) | 0.002 | 1.11 (0.99,1.25) | 0.076 | |
Model0, unadjusted model; Model1, adjusted by age, sex, tumour stage, and BMI (except for ALI); Model2, adjusted by age, sex tumour stage, BMI (except for ALI), KPS, PG‐SGA, surgery, radiotherapy, chemotherapy, smoking, and alcohol drinking.
95% CI, 95% confidence interval; AGR, albumin‐to‐globulin ratio; ALI, advanced lung cancer inflammation index; CONUT score, controlling nutritional status score; CRP, C‐reactive protein; GLR, glucose‐to‐lymphocyte ratio; GNRI, geriatric nutritional risk index; HR, hazard ratio; LCR, lymphocyte‐to‐C‐reactive protein ratio; LCS, lymphocyte‐to‐C‐reactive protein ratio score; mGNRI, modified geriatric nutritional risk index; mGPS, modified Glasgow prognostic score; NLR, neutrophil‐to‐lymphocyte ratio; NRI, nutritional risk index; PLR, platelet‐to‐lymphocyte ratio; PNI, prognostic nutritional index; SD, standard deviation; SII, neutrophil immune‐inflammation index; CAR, C‐reactive protein‐to‐albumin ratio.
Figure 2.
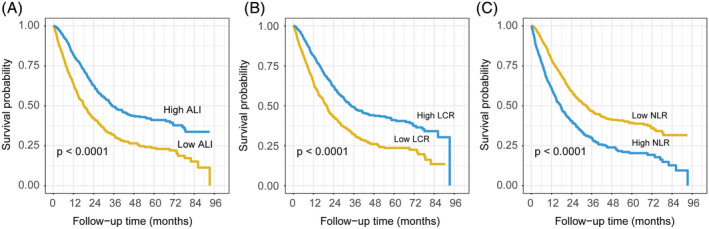
The Kaplan–Meier curves in patients with lung cancer of ALI (A), LCR (B), and NLR (C). ALI, advanced lung cancer inflammation index; LCR, lymphocyte‐to‐C‐reactive protein ratio; NLR, neutrophil‐to‐lymphocyte ratio.
The prognostic ability comparison of the inflammation/nutrition‐based indicators
A time‐ROC and C‐index were performed to compare the prognostic predictive capacity of 16 inflammation/nutrition‐based indicators in patients with lung cancer. Compared with the other inflammation/nutrition‐based indicators, the ALI showed the highest C‐index for OS in lung cancer patients at 1, 3, and 5 years: 0.617 (95% CI, 0.589–0.646), 0.607 (95% CI, 0.586–0.629), and 0.611 (95% CI, 0.591–0.631), respectively (Table 3). The ALI, LCR, and NLR were the Top 3 inflammation‐based indicators according to the 5‐year C‐index. Moreover, the ALI had a higher AUC value than the other inflammation/nutrition‐based indicators (Figure 3). Table S2 shows that the ALI, LCR, and NLR significantly contributed to the prognostic value of the TNM classification system. In different sub‐groups, the ALI had the highest C‐index in men and women, smokers and non‐smokers, aged <65 years, and SCLC lung cancer patients, as compared with the other indicators ( Tables S3–S6). In the sub‐group of lung cancer patients older than 65 years or had NSCLC, mGNRI had the highest C‐index (C‐index, 0.610 for age ≥65 and 0.639 for NSCLC) compared to the other indicators ( Tables S5 and S6).
Table 3.
The C‐index of 16 indicators for OS in patients with lung cancer
| C‐index (95% CI) | |||
|---|---|---|---|
| Indicators | 1‐year | 3‐year | 5‐year |
| ALI | 0.617 (0.589,0.646) | 0.607 (0.586,0.629) | 0.611 (0.591,0.631) |
| LCR | 0.577 (0.548,0.606) | 0.590 (0.568,0.611) | 0.604 (0.583,0.624) |
| NLR | 0.612 (0.583,0.640) | 0.598 (0.577,0.619) | 0.597 (0.577,0.618) |
| mGNRI | 0.564 (0.534,0.594) | 0.575 (0.553,0.596) | 0.597 (0.577,0.617) |
| CAR | 0.557 (0.527,0.587) | 0.577 (0.556,0.599) | 0.596 (0.576,0.617) |
| PNI | 0.580 (0.550,0.610) | 0.588 (0.567,0.609) | 0.593 (0.573,0.613) |
| NRI | 0.556 (0.526,0.587) | 0.573 (0.552,0.595) | 0.592 (0.572,0.612) |
| GNRI | 0.556 (0.526,0.587) | 0.573 (0.552,0.595) | 0.592 (0.572,0.612) |
| CRP | 0.555 (0.526,0.585) | 0.573 (0.551,0.595) | 0.592 (0.571,0.612) |
| CONUT | 0.589 (0.561,0.618) | 0.592 (0.571,0.613) | 0.586 (0.566,0.606) |
| SII | 0.581 (0.552,0.610) | 0.569 (0.548,0.590) | 0.576 (0.556,0.596) |
| mGPS | 0.556 (0.529,0.583) | 0.565 (0.546,0.584) | 0.575 (0.557,0.593) |
| GLR | 0.600 (0.570,0.629) | 0.586 (0.565,0.608) | 0.569 (0.549,0.590) |
| AGR | 0.525 (0.496,0.555) | 0.546 (0.525,0.568) | 0.567 (0.547,0.588) |
| LCS | 0.542 (0.517,0.567) | 0.539 (0.520,0.557) | 0.553 (0.536,0.571) |
| PLR | 0.567 (0.538,0.597) | 0.546 (0.525,0.568) | 0.545 (0.524,0.566) |
AGR, albumin‐to‐globulin ratio; ALI, advanced lung cancer inflammation index; CAR, C‐reactive protein‐to‐albumin ratio; CONUT score, controlling nutritional status score; CRP, C‐reactive protein; GLR, glucose‐to‐lymphocyte ratio; GNRI, geriatric nutritional risk index; LCR, lymphocyte‐to‐C‐reactive protein ratio; LCS, lymphocyte‐to‐C‐reactive protein ratio score; mGNRI, modified geriatric nutritional risk index; mGPS, modified Glasgow prognostic score; NLR, neutrophil‐to‐lymphocyte ratio; NRI, nutritional risk index; OS, overall survival; PLR, platelet‐to‐lymphocyte ratio; PNI, prognostic nutritional index; SII, systemic immune‐inflammation index.
Figure 3.
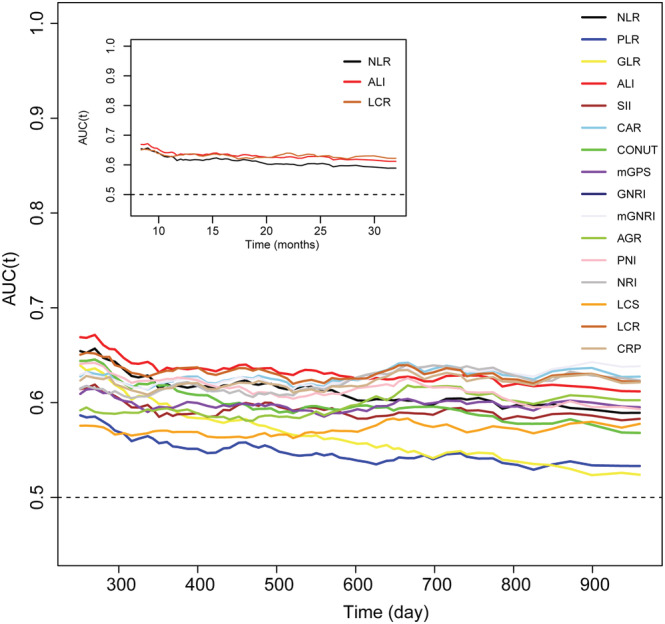
The time‐dependent ROC of inflammation and nutrition‐relative indicators for diagnosing overall survival in patients with lung cancer. AGR, albumin‐to‐globulin ratio; ALI, advanced lung cancer inflammation index; CAR, neutrophil‐to‐lymphocyte ratio; CONUT score, controlling nutritional status score; CRP, C‐reactive protein; GLR, glucose‐to‐lymphocyte ratio; GNRI, geriatric nutritional risk index; LCR, lymphocyte‐to‐C‐reactive protein ratio; LCS, lymphocyte‐to‐C‐reactive protein ratio score; mGNRI, modified geriatric nutritional risk index; mGPS, modified Glasgow prognostic score; NLR, neutrophil‐to‐lymphocyte ratio; NRI, nutritional risk index; PLR, platelet‐to‐lymphocyte ratio; PNI, prognostic nutritional index; SII, neutrophil immune‐inflammation index.
Analysis of the Top 3 indicators and clinico‐pathological characteristics in lung cancer patients
Overall, the ALI, LCR, and NLR were the Top three inflammation/nutrition‐based indicators for predicting the prognosis of patients with lung cancer. The baseline characteristics of the lung cancer patients stratified by high/low ALI, LCR, and NLR are shown in Tables S7–S9. A forest plot of the results of the sub‐group analyses of the ALI, LCR, and NLR in patients with lung cancer (Figure 4) showed that a low ALI, high LCR, and high NLR were risk factors for mortality in lung cancer patients who were younger or older than 65 years, men, women, non‐smokers, had NSCLC, obesity (BMI ≥ 28 kg/m2), or TNM Stage III. Interestingly, the smoking status (P for interaction = 0.004), lung cancer types (P for interaction < 0.001) of the patients, and a high NLR had interactive effects. Moreover, patients with lung cancer had an increased NLR and decreased ALI and LCR along with the elevated TNM stage ( Figure S3). Figures S4 and S5 showed the Kaplan–Meier curves of the ALI, LCR, and NLR in different sub‐groups of lung cancer patients stratified by sex, smoking status, TNM stage, and pathological type. Patients with lung cancer with a low ALI, low LCR, or high NLR had poor OS, even in 30‐day outcome (Figure 5).
Figure 4.
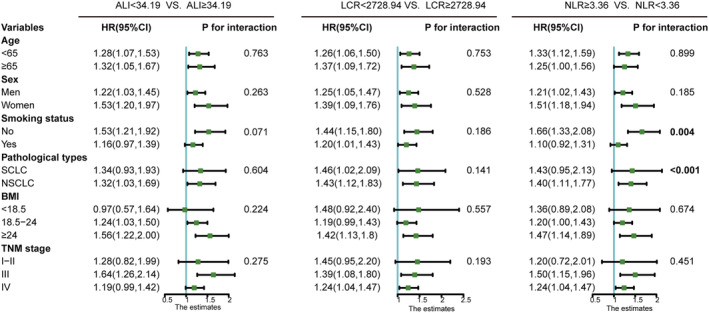
The sub‐group analysis of ALI, LCR, and NLR in patients with lung cancer. The adjusted factors include age, sex tumour stage, BMI (except for ALI), KPS, PG‐SGA, surgery, radiotherapy, chemotherapy, smoking and alcohol drinking. 95% CI, 95% confidence interval; ALI, advanced lung cancer inflammation index; BMI, body mass index; HR, hazard ratio; LCR, lymphocyte‐to‐C‐reactive protein ratio; NLR, neutrophil‐to‐lymphocyte ratio; NSCLC, non‐small cell lung cancer; SCLC, small cell lung cancer.
Figure 5.
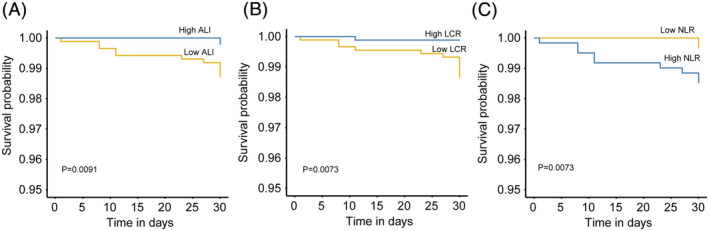
The Kaplan–Meier curves of 30‐day outcome in patients with lung cancer. ALI, advanced lung cancer inflammation index; LCR, lymphocyte‐to‐C‐reactive protein ratio; NLR, neutrophil‐to‐lymphocyte ratio.
Discussion
Increasing evidence has indicated that the inflammatory/nutrition‐based indicators are reliable predictors of the OS of patients with cancer, but the optimal indicator for lung cancer patients is not clear. Our study used a large cohort to assessed and compared 16 inflammation/nutrition‐based indicators and found that the ALI was stably and consistently discriminative in risk stratification across most sub‐groups of lung cancer patients. In addition, we found that the mGNRI was the preferable indicator in the sub‐groups of patients with lung cancer who were aged ≥65 years or had NSCLC. Among patients with SCLC, the GLR was a better prognostic predictor than the other indicators.
Previous studies reported that LCR, 26 ALI, NLR, PLR, 6 SII, 27 CAR, 28 mGPS, 29 CONUT score, 30 AGR, 14 PNI, 31 NRI, GNRI, 32 and CRP 7 were useful as predictors of the prognosis of patients with lung cancer. Consistent with previous studies, our study found that PLR, ALI, NLR, SII, CAR, CONUT score, mGPS, GNRI, AGR, PNI, NRI, LCR, and CRP were all associated with OS in univariate analysis; each of these indicators except the PLR was an independent prognostic indicators of lung cancer patients and that GLR, mGNRI, and LCS, which were not examined in previous study, were also independent predictors of prognosis in patients with lung cancer. One study reported that the NLR, PLR, and ALI were associated with the OS of patients with lung cancer 6 ; however, the comparisons of these three indicators were not performed in lung cancer patients in their study. A recent study reported that CAR has a stronger effect on prediction of a postoperative poor prognosis for NSCLC patients than GPS and mGPS. 33 Our study also identified that CAR and mGPS were independent prognostic biomarkers in lung cancer patients; however, the prediction effect of ALI on the poor prognosis for lung cancer was better than CAR among 16 inflammation/nutrition‐based indicators. In our study, the ALI displayed the best predictive performance for prognosis in patients with lung cancer among the inflammation/nutrition‐based indicators. ALI is an objective, easy‐to‐use, and simplified approach for facilitating the timely identification of lung cancer patients in clinical practice. The results need to be confirmed by more prospective studies.
The ALI was developed initially for assessing the degree of systemic inflammation at the time of diagnosis in patients with metastatic NSCLC. The ALI is a composite index derived from three factors: BMI, serum albumin levels, and NLR. The BMI, albumin levels, and NLR are, respectively, anthropometric indicator, nutrition‐related, and inflammation‐related indicators. An independent protective association was observed between obesity and lung cancer‐related mortality. 34 The serum albumin level and NLR are also important prognostic factors for OS in patients with lung cancer. 35 Compared with the other indicators, the ALI is the only indicator that covers anthropometric, nutritional, and inflammatory factors associated with the prognosis of lung cancer. This may be one reason why the ALI had a better predictive performance in the prognosis of lung cancer than the other indicators.
Bouillanne et al. reported that the mGNRI was a possible predictor of prognosis in elderly patients with heart failure; several studies have reported that the mGNRI is also a useful predictor of prognosis in malignancies. 24 We found that the mGNRI was a useful independent prognostic factor in patients with lung cancer, and it had the best predictive significance in the prognosis of lung cancer patients over 65 years of age. In addition, the mGNRI had the highest C‐index in patients with NSCLC; the GLR performed the best in patients with SCLC. Because nearly half of the patients failed to undergo pathological classification, this may not be representative and needs to be verified in a large‐population study. Although the mGNRI and GLR had the highest C‐index for three sub‐groups, the ALI had the best predictive significance in the prognosis of lung cancer in general. These biomarkers, the ALI, mGNRI, and GLR represent convenient and inexpensive indicators that can be used to evaluate the status of systemic inflammation or nutrition. These inflammation/nutrition‐based indicators may allow for the early stratification of patients with lung cancer to optimize treatment.
Previous study has reported that high NLR is also a risk factor of mortality in patients with lung cancer in a low‐risk population, such as never‐smokers. 36 It is estimated that approximately 25% of all lung cancer cases are observed in never‐smokers. 37 In our study, 39.3% of lung cancer were non‐smokers. In fact, these lung cancer patients in non‐smokers are considered as a special disease with various different tumorigenic mode, clinico‐pathology, and natural history and is the seventh leading cause of cancer‐related deaths. 38 Intriguingly, our study observed an interactive effect between high NLR and smoking status in lung cancer patients, indicating that those lung cancer patients in non‐smokers with high NLR had higher risk of mortality. In our study, the NLR level had no significant difference in different sex and smoking status (data were not shown). However, female patients in those lung cancer patients with high NLR in non‐smokers had 64% level count ( Table S10). Previous studies showed that female lung cancer in non‐smokers had more gene mutations 39 and tend to be affected by second‐hand smoke. 40 Second‐hand smoke is one of the mortality risks of lung cancer patients in non‐smokers. 37 Our study indicated that inflammation may be another risk factor of the mortality in non‐smoker lung cancer patients. However, the underlying mechanism needs to be identified in further study. In addition, we also found that the NLR and pathologic type of lung cancer had a significant interactive effect. Those patients with NSCLC combined with high NLR had a higher risk of mortality. The different pathology types of lung cancer may lead to the different sensitivity or mechanism of inflammation, resulting in different degrees of mortality risk.
This study had several limitations. First, the pathological classification data of some patients with lung cancer were not determined, and the results for different lung cancer sub‐groups need to be further verified. Second, the limitation of relevant data on the targeted therapy and immunotherapy and the use of anti‐inflammatory drugs in this study may have affected the results. However, this study cannot exclude the influence of this factor on the results, which should be verified in more rigorous cohort studies in the future. Third, this study lacked continuous data. If there were dynamic changes in the indicators before or after the intervention, the results might be more convincing; whether survival outcomes are improved after the corresponding intervention needs to be verified by future studies.
In conclusion, compared with the other indicators, the ALI showed the best performance in predicting the prognosis of patients with lung cancer in general and in most sub‐groups. The evaluation of the ALI could identify lung cancer patients at risk of a poor prognosis and be a useful prognostic marker in clinical practice.
Funding
This work was financially supported by National Key Research and Development Program (No. 2017YFC1309200) and Beijing Municipal Science and Technology Commission (SCW2018‐06) to Dr Hanping Shi and General Surgery Clinical Medical Center of Yunnan Province (No. ZX20190303) to Dr Kunhua Wang.
Conflict of interest
None.
Supporting information
Figure S1. The unadjusted and adjusted restricted cubic spline of other inflammation and nutrition‐relative indicators in patients with lung cancer. The adjusted factors include age, sex tumor stage, BMI, KPS, PG‐SGA, surgery, radiotherapy, chemotherapy, smoking and alcohol drinking. ALI, advanced lung cancer inflammation index; LCR, lymphocyte C‐reactive protein ratio; NLR, neutrophil to lymphocyte ratio; mGNRI, modified geriatric nutritional risk index; CAR, C‐reactive protein‐albumin ratio; PNI, prognostic nutritional index; NRI, nutritional risk index; GNRI, geriatric nutritional risk index; CRP, C‐reactive protein; CONUT score, controlling nutritional status score; SII, neutrophil immune‐inflammation index; mGPS, modified Glasgow prognostic score; GLR, glucose to lymphocyte ratio; AGR, albumin‐globulin ratio; LCS, lymphocyte‐c‐reactive.
Figure S2. The Kaplan‐–Meier curves of the other thirteen indicators in patients with lung cancer. mGNRI, modified geriatric nutritional risk index; CAR, C‐reactive protein‐albumin ratio; PNI, prognostic nutritional index; NRI, nutritional risk index; GNRI, geriatric nutritional risk index; CRP, C‐reactive protein; CONUT score, controlling nutritional status score; SII, neutrophil immune‐inflammation index; mGPS, modified Glasgow prognostic score; GLR, glucose to lymphocyte ratio; AGR, albumin‐globulin ratio; LCS, lymphocyte‐c‐reactive protein ratio score; PLR, platelet to lymphocyte ratio.
Figure S3. The scatterplot of ALI, LCR and NLR in different TNM stage. ALI, advanced lung cancer inflammation index; LCR, lymphocyte C‐reactive protein ratio; NLR, neutrophil to lymphocyte ratio. TNM stage I was regarded as the reference group; ns, no significant; *** P < 0.0001.
Figure S4. The Kaplan‐–Meier curves of the ALI, LCR and NLR in lung cancer patients stratified by sex and smoking status: (A) male lung cancer patients, (B) female lung cancer patients, (C) smoking; (D) non‐smoking. ALI, advanced lung cancer inflammation index; LCR, lymphocyte C‐reactive protein ratio; NLR, neutrophil to lymphocyte ratio.
Figure S5. The Kaplan‐–Meier curves of the ALI, LCR and NLR in patients with lung cancer stratified by TNM stages and pathological types: (A) SCLC, (B) NSCLC, (C) TNM stage I‐II; (D) TNM stage III; (E) TNM stage IV. ALI, advanced lung cancer inflammation index; LCR, lymphocyte C‐reactive protein ratio; NLR, neutrophil to lymphocyte ratio ALI, advanced lung cancer inflammation index; LCR, lymphocyte C‐reactive protein ratio; NLR, neutrophil to lymphocyte ratio.
Table S1. Calculation methods of combination in each nutrition/inflammation‐based indicator.
Table S2. The C‐index of different prognostic models on 1772 lung cancer patients.
Table S3. The C‐index of fifteen indicators for OS in patients with lung cancer stratified by sex.
Table S4. The C‐index of fifteen indicators for OS in patients with lung cancer stratified by smoking status.
Table S5. The C‐index of fifteen indicators for OS in patients with lung cancer stratified by age.
Table S6. The C‐index of fifteen indicators for OS in patients with lung cancer stratified by pathological type.
Table S7. Baseline characteristics stratified by ALI.
Table S8. Baseline characteristics stratified by LCR.
Table S9. Baseline characteristics stratified by NLR.
Table S10. Baseline characteristics stratified by smoking status in lung cancer patients with high NLR.
Acknowledgements
We would like to express our sincere thanks to the INSCOC project members for their substantial work on data collection and patient follow‐up.
Song M., Zhang Q., Song C., Liu T., Zhang X., Ruan G., Tang M., Xie H., Zhang H., Ge Y., Li X., Zhang K., Yang M., Li Q., Liu X., Lin S., Xu Y., Xu H., Wang K., Li W., and Shi H. (2022) The advanced lung cancer inflammation index is the optimal inflammatory biomarker of overall survival in patients with lung cancer, Journal of Cachexia, Sarcopenia and Muscle, 13, 2504–2514, 10.1002/jcsm.13032
Mengmeng Song, Qi Zhang, and Chunhua Song contributed equally to the present study.
References
- 1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209–249. [DOI] [PubMed] [Google Scholar]
- 2. World cancer report 2020. 2021.
- 3. Früh M, de Ruysscher D, Popat S, Crinò L, Peters S, Felip E, et al. Small‐cell lung cancer (SCLC): ESMO clinical practice guidelines for diagnosis, treatment and follow‐up. Ann Oncol: Off J Eur Soc Med Oncol 2013;24:vi99–vi105. [DOI] [PubMed] [Google Scholar]
- 4. Le‐Rademacher J, Lopez C, Wolfe E, Foster NR, Mandrekar SJ, Wang X, et al. Weight loss over time and survival: a landmark analysis of 1000+ prospectively treated and monitored lung cancer patients. J Cachexia Sarcopenia Muscle 2020;11:1501–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Burtin C, Bezuidenhout J, Sanders KJC, Dingemans AC, Schols A, Peeters STH, et al. Handgrip weakness, low fat‐free mass, and overall survival in non‐small cell lung cancer treated with curative‐intent radiotherapy. J Cachexia Sarcopenia Muscle 2020;11:424–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mandaliya H, Jones M, Oldmeadow C, Nordman II. Prognostic biomarkers in stage IV non‐small cell lung cancer (NSCLC): neutrophil to lymphocyte ratio (NLR), lymphocyte to monocyte ratio (LMR), platelet to lymphocyte ratio (PLR) and advanced lung cancer inflammation index (ALI). Transl Lung Cancer Res 2019;8:886–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pastorino U, Morelli D, Leuzzi G, Gisabella M, Suatoni P, Taverna F, et al. Baseline and postoperative C‐reactive protein levels predict mortality in operable lung cancer. Eur J Cancer 2017;79:90–97. [DOI] [PubMed] [Google Scholar]
- 8. Galvano A, Peri M, Guarini AA, Castiglia M, Grassadonia A, De Tursi M, et al. Analysis of systemic inflammatory biomarkers in neuroendocrine carcinomas of the lung: prognostic and predictive significance of NLR, LDH, ALI, and LIPI score. Ther Adv Med Oncol 2020;12:1758835920942378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. He X, Zhou T, Yang Y, Hong S, Zhan J, Hu Z, et al. Advanced lung cancer inflammation index, a new prognostic score, predicts outcome in patients with small‐cell lung cancer. Clin Lung Cancer 2015;16:e165–e171. [DOI] [PubMed] [Google Scholar]
- 10. Jafri SH, Shi R, Mills G. Advance lung cancer inflammation index (ALI) at diagnosis is a prognostic marker in patients with metastatic non‐small cell lung cancer (NSCLC): A retrospective review. BMC Cancer 2013;13:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hu Z, Wu W, Zhang X, Li P, Zhang H, Wang H, et al. Advanced lung cancer inflammation index is a prognostic factor of patients with small‐cell lung cancer following surgical resection. Cancer Manag Res 2021;13:2047–2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yang R, Chang Q, Meng X, Gao N, Wang W. Prognostic value of systemic immune‐inflammation index in cancer: A meta‐analysis. J Cancer 2018;9:3295–3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang JR, Xu JY, Chen GC, Yu N, Yang J, Zeng DX, et al. Post‐diagnostic C‐reactive protein and albumin predict survival in Chinese patients with non‐small cell lung cancer: A prospective cohort study. Sci Rep 2019;9:8143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang Y, Li S, Hu X, Wang Y, Wu Y, Li P, et al. The prognostic value of serum albumin‐globulin ratio in early‐stage non‐small cell lung cancer: A retrospective study. Cancer Manag Res 2019;11:3545–3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lindenmann J, Fink‐Neuboeck N, Taucher V, Pichler M, Posch F, Brcic L, et al. Prediction of postoperative clinical outcomes in resected stage I non‐small cell lung cancer focusing on the preoperative Glasgow prognostic score. Cancer 2020;12:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hino H, Saito T, Matsui H, Taniguchi Y, Murakawa T. Utility of geriatric nutritional risk index in patients with lung cancer undergoing surgery. Eur J Cardiothorac Surg 2020;58:775–782. [DOI] [PubMed] [Google Scholar]
- 17. Ramos R, Nadal E, Peiró I, Masuet‐Aumatell C, Macia I, Rivas F, et al. Preoperative nutritional status assessment predicts postoperative outcomes in patients with surgically resected non‐small cell lung cancer. Eur J Surg Oncol 2018;44:1419–1424. [DOI] [PubMed] [Google Scholar]
- 18. Okada S, Shimada J, Kato D, Tsunezuka H, Teramukai S, Inoue M. Clinical significance of prognostic nutritional index after surgical treatment in lung cancer. Ann Thorac Surg 2017;104:296–302. [DOI] [PubMed] [Google Scholar]
- 19. Lee SC, Lee JG, Lee SH, Kim EY, Chang J, Kim DJ, et al. Prediction of postoperative pulmonary complications using preoperative controlling nutritional status (CONUT) score in patients with resectable non‐small cell lung cancer. Sci Rep 2020;10:12385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhong A, Cheng CS, Kai J, Lu R, Guo L. Clinical significance of glucose to lymphocyte ratio (GLR) as a prognostic marker for patients with pancreatic cancer. Front Oncol 2020;10:520330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Navarro J, Kang I, Hwang HK, Yoon DS, Lee WJ, Kang CM. Glucose to lymphocyte ratio as a prognostic marker in patients with resected pT2 gallbladder cancer. J Surg Res 2019;240:17–29. [DOI] [PubMed] [Google Scholar]
- 22. Okugawa Y, Toiyama Y, Yamamoto A, Shigemori T, Ide S, Kitajima T, et al. Lymphocyte‐C‐reactive protein ratio as promising new marker for predicting surgical and oncological outcomes in colorectal cancer. Ann Surg 2020;272:342–351. [DOI] [PubMed] [Google Scholar]
- 23. Okugawa Y, Toiyama Y, Yamamoto A, Shigemori T, Ichikawa T, Yin C, et al. Lymphocyte‐to‐C‐reactive protein ratio and score are clinically feasible nutrition‐inflammation markers of outcome in patients with gastric cancer. Clin Nutr (Edinburgh, Scotland) 2020;39:1209–1217. [DOI] [PubMed] [Google Scholar]
- 24. Kouzu K, Tsujimoto H, Sugasawa H, Ishibashi Y, Hase K, Kishi Y, et al. Modified geriatric nutrition risk index as a prognostic predictor of esophageal cancer. Esophagus 2021;18:278–287. [DOI] [PubMed] [Google Scholar]
- 25. Song C, Cao J, Zhang F, Wang C, Guo Z, Lin Y, et al. Nutritional risk assessment by scored patient‐generated subjective global assessment associated with demographic characteristics in 23,904 common malignant tumors patients. Nutr Cancer 2019;71:50–60. [DOI] [PubMed] [Google Scholar]
- 26. He Y, Gong R, Peng KW, Liu LZ, Sun LY, Wang HY. Lymphocyte‐to‐C‐reactive protein ratio is a potential new prognostic biomarker for patients with lung cancer. Biomark Med 2020;14:717–726. [DOI] [PubMed] [Google Scholar]
- 27. Gao Y, Zhang H, Li Y, Wang D, Ma Y, Chen Q. Preoperative increased systemic immune‐inflammation index predicts poor prognosis in patients with operable non‐small cell lung cancer. Clin Chim Acta 2018;484:272–277. [DOI] [PubMed] [Google Scholar]
- 28. Zhou T, Zhan J, Hong S, Hu Z, Fang W, Qin T, et al. Ratio of C‐reactive protein/albumin is an inflammatory prognostic score for predicting overall survival of patients with small‐cell lung cancer. Sci Rep 2015;5:10481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fan H, Shao ZY, Xiao YY, Xie ZH, Chen W, Xie H, et al. Comparison of the Glasgow prognostic score (GPS) and the modified Glasgow prognostic score (mGPS) in evaluating the prognosis of patients with operable and inoperable non‐small cell lung cancer. J Cancer Res Clin Oncol 2016;142:1285–1297. [DOI] [PubMed] [Google Scholar]
- 30. Liu XY, Zhang X, Zhang Q, Xie HL, Ruan GT, Liu T, et al. Value of the controlling nutritional status score in predicting the prognosis of patients with lung cancer: A multicenter, retrospective study. JPEN J Parenter Enteral Nutr 2021. [DOI] [PubMed] [Google Scholar]
- 31. Park S, Ahn HJ, Yang M, Kim JA, Kim JK, Park SJ. The prognostic nutritional index and postoperative complications after curative lung cancer resection: A retrospective cohort study. J Thorac Cardiovasc Surg 2020;160:276–285.e1. [DOI] [PubMed] [Google Scholar]
- 32. Wang H, Li C, Yang R, Jin J, Liu D, Li W. Prognostic value of the geriatric nutritional risk index in non‐small cell lung cancer patients: A systematic review and meta‐analysis. Front Oncol 2021;11:794862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Matsubara T, Takamori S, Haratake N, Fujishita T, Toyozawa R, Ito K, et al. Identification of the best prognostic marker among immunonutritional parameters using serum C‐reactive protein and albumin in non‐small cell lung cancer. Ann Surg Oncol 2021;28:3046–3054. [DOI] [PubMed] [Google Scholar]
- 34. Gupta A, Majumder K, Arora N, Mayo HG, Singh PP, Beg MS, et al. Premorbid body mass index and mortality in patients with lung cancer: A systematic review and meta‐analysis. Lung Cancer (Amsterdam, Netherlands) 2016;102:49–59. [DOI] [PubMed] [Google Scholar]
- 35. Miura K, Hamanaka K, Koizumi T, Kitaguchi Y, Terada Y, Nakamura D, et al. Clinical significance of preoperative serum albumin level for prognosis in surgically resected patients with non‐small cell lung cancer: Comparative study of normal lung, emphysema, and pulmonary fibrosis. Lung Cancer (Amsterdam, Netherlands) 2017;111:88–95. [DOI] [PubMed] [Google Scholar]
- 36. Kang J, Chang Y, Ahn J, Oh S, Koo DH, Lee YG, et al. Neutrophil‐to‐lymphocyte ratio and risk of lung cancer mortality in a low‐risk population: A cohort study. Int J Cancer 2019;145:3267–3275. [DOI] [PubMed] [Google Scholar]
- 37. Pallis AG, Syrigos KN. Lung cancer in never smokers: Disease characteristics and risk factors. Crit Rev Oncol Hematol 2013;88:494–503. [DOI] [PubMed] [Google Scholar]
- 38. Rivera GA, Wakelee H. Lung cancer in never smokers. Adv Exp Med Biol 2016;893:43–57. [DOI] [PubMed] [Google Scholar]
- 39. Dacic S. Molecular genetic testing for lung adenocarcinomas: a practical approach to clinically relevant mutations and translocations. J Clin Pathol 2013;66:870–874. [DOI] [PubMed] [Google Scholar]
- 40. Clément‐Duchêne C, Vignaud JM, Stoufflet A, Bertrand O, Gislard A, Thiberville L, et al. Characteristics of never smoker lung cancer including environmental and occupational risk factors. Lung Cancer (Amsterdam, Netherlands) 2010;67:144–150. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. The unadjusted and adjusted restricted cubic spline of other inflammation and nutrition‐relative indicators in patients with lung cancer. The adjusted factors include age, sex tumor stage, BMI, KPS, PG‐SGA, surgery, radiotherapy, chemotherapy, smoking and alcohol drinking. ALI, advanced lung cancer inflammation index; LCR, lymphocyte C‐reactive protein ratio; NLR, neutrophil to lymphocyte ratio; mGNRI, modified geriatric nutritional risk index; CAR, C‐reactive protein‐albumin ratio; PNI, prognostic nutritional index; NRI, nutritional risk index; GNRI, geriatric nutritional risk index; CRP, C‐reactive protein; CONUT score, controlling nutritional status score; SII, neutrophil immune‐inflammation index; mGPS, modified Glasgow prognostic score; GLR, glucose to lymphocyte ratio; AGR, albumin‐globulin ratio; LCS, lymphocyte‐c‐reactive.
Figure S2. The Kaplan‐–Meier curves of the other thirteen indicators in patients with lung cancer. mGNRI, modified geriatric nutritional risk index; CAR, C‐reactive protein‐albumin ratio; PNI, prognostic nutritional index; NRI, nutritional risk index; GNRI, geriatric nutritional risk index; CRP, C‐reactive protein; CONUT score, controlling nutritional status score; SII, neutrophil immune‐inflammation index; mGPS, modified Glasgow prognostic score; GLR, glucose to lymphocyte ratio; AGR, albumin‐globulin ratio; LCS, lymphocyte‐c‐reactive protein ratio score; PLR, platelet to lymphocyte ratio.
Figure S3. The scatterplot of ALI, LCR and NLR in different TNM stage. ALI, advanced lung cancer inflammation index; LCR, lymphocyte C‐reactive protein ratio; NLR, neutrophil to lymphocyte ratio. TNM stage I was regarded as the reference group; ns, no significant; *** P < 0.0001.
Figure S4. The Kaplan‐–Meier curves of the ALI, LCR and NLR in lung cancer patients stratified by sex and smoking status: (A) male lung cancer patients, (B) female lung cancer patients, (C) smoking; (D) non‐smoking. ALI, advanced lung cancer inflammation index; LCR, lymphocyte C‐reactive protein ratio; NLR, neutrophil to lymphocyte ratio.
Figure S5. The Kaplan‐–Meier curves of the ALI, LCR and NLR in patients with lung cancer stratified by TNM stages and pathological types: (A) SCLC, (B) NSCLC, (C) TNM stage I‐II; (D) TNM stage III; (E) TNM stage IV. ALI, advanced lung cancer inflammation index; LCR, lymphocyte C‐reactive protein ratio; NLR, neutrophil to lymphocyte ratio ALI, advanced lung cancer inflammation index; LCR, lymphocyte C‐reactive protein ratio; NLR, neutrophil to lymphocyte ratio.
Table S1. Calculation methods of combination in each nutrition/inflammation‐based indicator.
Table S2. The C‐index of different prognostic models on 1772 lung cancer patients.
Table S3. The C‐index of fifteen indicators for OS in patients with lung cancer stratified by sex.
Table S4. The C‐index of fifteen indicators for OS in patients with lung cancer stratified by smoking status.
Table S5. The C‐index of fifteen indicators for OS in patients with lung cancer stratified by age.
Table S6. The C‐index of fifteen indicators for OS in patients with lung cancer stratified by pathological type.
Table S7. Baseline characteristics stratified by ALI.
Table S8. Baseline characteristics stratified by LCR.
Table S9. Baseline characteristics stratified by NLR.
Table S10. Baseline characteristics stratified by smoking status in lung cancer patients with high NLR.


