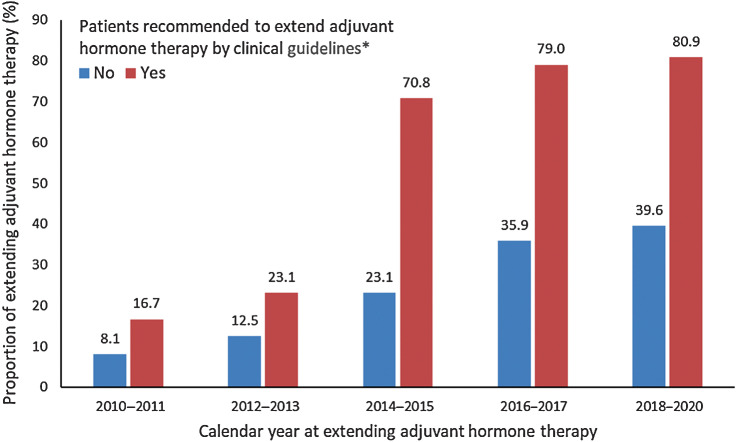
Figure 3.
Prevalence of extended therapy among patients who finished 5-year adjuvant hormone therapy, by whether recommended to extend therapy by the Swedish National Clinical Guideline, 2010 to 2020. Note: 365 of 504 patients who met the criteria to extend adjuvant hormone therapy in the clinical guideline extended their therapy after finishing 5-year adjuvant hormone therapy, and 993 of 3,727 patients who did not meet therapy extension criteria extended their therapy after finishing 5-year adjuvant hormone therapy. *, Patients with the following characteristics are recommended to extend adjuvant hormone therapy beyond 5 years by the Swedish National Clinical Guideline: during 2010 to 2014, postmenopausal women with lymph node–positive tumor and treated with tamoxifen during the first 5 years, which is consistent with ASCO guideline 2010 and ESMO guideline 2010; during 2015 to 2017, further including premenopause women with lymph node–positive tumor and treated with tamoxifen during the first 5 years, which is consistent with ASCO guideline 2014 and ESMO guideline 2015; during 2018 to 2020, further including postmenopause women with lymph node–positive tumor and treated with aromatase inhibitors during the first 5 years, which is consistent with ASCO guideline 2018.