Abstract
Alcohol-use-disorders are chronic relapsing illnesses, often co-morbid with anxiety. We have previously shown using the “drinking-in-the-dark” model in mice that the stimulation of the serotonin receptor 1A (5-HT1A) reduces ethanol binge-drinking behaviour and withdrawal-induced anxiety. The 5-HT1A receptor is located either on Raphe neurons as autoreceptors, or on target neurons as heteroreceptors. By combining a pharmacological approach with biased agonists targeting the 5-HT1A auto- or heteroreceptor and a chemogenetic approach (DREADDs), here we identified that ethanol-binge drinking behaviour is dependent on 5-HT1A autoreceptors and 5-HT neuronal function, with a transition from DRN-dependent regulation of short-term (6 weeks) ethanol intake, to MRN-dependent regulation after longer ethanol exposure (12 weeks). We further identified a serotonergic microcircuit (5-HTMRN→DG) originating from the MRN and projecting to the dentate gyrus (DG) of the hippocampus, that is specifically affected by, and modulates long-term ethanol consumption. The present study indicates that targeting Raphe nuclei 5-HT1A autoreceptors with agonists might represent an innovative pharmacotherapeutic strategy to combat alcohol abuse.
Subject terms: Neuroscience, Physiology
Main
Alcohol-use-disorders (AUDs) are chronic relapsing illnesses, with alcohol often self-medicated to cope with the distressing symptoms of various mental health issues. The COVID-19 pandemic led to greater reports of stress- and fear-related mental health issues, possibly contributing to increases in worldwide alcohol consumption [1–5]. Given that the brain serotonin (5-Hydroxytryptamine, 5-HT) system is closely involved in the stress responses to social isolation [6, 7], fear [8, 9], anxiety [9, 10] and depression [11], we hypothesized that alterations in brain 5-HT neurotransmission contributes to the reinforcement of alcohol seeking/drinking behaviour. Research studies have shown that manipulation of the neural activity of 5-HT neurons from the dorsal or median Raphe nuclei (DRN or MRN, respectively) alters anxiety-like behaviour, antidepressant-like and anti-impulsive-like effects [12–15], arousal states [16, 17], sleep cycles [18, 19], response to rewarding or aversive stimuli [20–23], social behaviour and aggression [16, 24]. Since most of these behaviours are also affected by alcohol consumption, it is likely that 5-HT neuroplasticity plays a role in the maintenance of long-term alcohol consumption [25].
Previous studies from our laboratory and others have revealed that long-term alcohol consumption alters the expression and function of serotonin 1A (5-HT1A) autoreceptors (i.e., those located on Raphe nuclei) and heteroreceptors (i.e., those located on projection brain regions) [26–28], as well as the morphology [29] and function [30] of Raphe nuclei 5-HT neurons. We further demonstrated that a chronic treatment with pindolol or tandospirone, partial and more efficacious 5-HT1A receptor agonists, respectively, reduces ethanol intake, prevents withdrawal-induced anxiety-like behaviour and reverses the deficits in hippocampal neurogenesis elicited by long-term ethanol intake in mice [28, 31]. However, the specific contribution of 5-HT1A auto- vs heteroreceptors, and the role played by Raphe nuclei 5-HT neurotransmission in alcohol drinking behaviour is less well understood. In the present study, we used NLX-112 (a.k.a. befiradol or F13640), a highly selective unbiased 5-HT1A agonist (i.e., with no marked preference for auto- vs heteroreceptors), or biased agonists that preferentially target 5-HT1A autoreceptors (F13714) or heteroreceptors (NLX-101, a.k.a. F5599) [32–34] to determine whether 5-HT1A auto- or heteroreceptors mediate the effect of 5-HT1A receptor agonists on the reduction of ethanol intake. The molecular basis for the auto/heteroreceptor selectivity of these 5-HT1A receptor biased agonists appears to be related to preferential activation of specific G-protein subtypes in different brain regions [35–37]. Evidence supporting the differential coupling to G-proteins and consequent differential activation of downstream signalling cascades has been reviewed extensively [38]. We identified 5-HT1A autoreceptors as the receptor subpopulation mediating both short- and long-term ethanol intake. Brain cannulation and local microinjections of these agonists allowed us to identify which nucleus, DRN or MRN, mediates the 5-HT1A receptor-dependent reduction in ethanol intake following short- vs long-term exposure in the “drinking in the dark” model in mice. We further used chemogenetic manipulation of pet1-5-HT neuron activity with CRE-dependent Designer Receptors Exclusively Activated by Designer Drugs (DREADDs), in combination with systemic or local injections of the designer drug Clozapine-N-Oxide (CNO) to identify the serotonergic circuits that control ethanol intake following long-term exposure. Lastly, we also assessed the systemic effects of NLX-112 and F13714, and intracranial effects (Raphe nuclei microinjection) of the latter on food (chow) and water intake, as well as on locomotor activity in naïve mice, to control for unspecific effects.
Using this double pharmacological and chemogenetic strategy, we discovered that the 5-HT neuronal circuits involved in ethanol consumption switched after long-term exposure, from DRN to MRN, and suggests that selective 5-HT1A receptor biased agonists targeting those particular circuits might represent viable pharmacotherapeutics for the treatment of AUDs.
Methods
Animals and housing
Five-week-old male C57BL/6 J mice (ARC, WA, Australia) or hemizygous pet1-CRE mice [39] (Strain number: 012712, B6.Cg-Tg(Fev-cre)1Esd/J, JaxMice, USA) were individually housed under reverse light cycle conditions (lights off from 9:00 am to 9:00 pm) in a climate-controlled room (22 °C, 50 % humidity) with ad libitum access to food and filtered tap water. For each experiment involving animals, sample size was chosen according to previous studies from the laboratory. Following 1 week of habituation to the housing conditions, mice were offered alcohol during drinking-in-the-dark sessions. The Pet1-CRE+/0 allele was detected by PCR with the following primers: 5ʹ- CTTCTGTCCGTTTGCCGGTCGTGG / TTTTGCACGTTCACC GGCATCAACG -3ʹ that amplified a band of 264 bp as previously described [40]. All experimental procedures were approved by The University of Queensland and The Queensland University of Technology Animal Ethics Committees and complied with the policies and regulations regarding animal experimentation and other ethical matters, in accordance with the Queensland Government Animal Research Act 2001, associated Animal Care and Protection Regulations (2002 and 2008), as well as the Australian Code for the Care and Use of Animals for Scientific Purposes, 8th Edition (National Health and Medical Research Council, 2013).
“Drinking-in-the-dark” ethanol intake model
We adapted the “Drinking-In-the-Dark” (DID) model of binge-like alcohol [41] or sucrose consumption with long-term exposure as previously described [27, 28, 31]. Mice were given access to one bottle of 20% (v/v) alcohol for a 2 h period (12 pm to 2 pm), 3 h into the dark cycle, Monday to Friday. Filtered water was available at all other times. The alcohol solution was presented in 50 ml plastic falcon tubes (Corning Centristar, NY, USA) fitted with rubber stoppers and a 6.35 cm stainless-steel sipper tube with double ball bearings. Alcohol containing tubes were weighed prior to and 2 h after presentation. For DREADD/DG-CNO injection, alcohol containing tube were weighed prior to, and 30 min and 2 h after presentation. Mouse weights were measured daily for 12 weeks to calculate the adjusted g/kg intake.
Drugs
Drug administration occurred at 6 and 12 weeks in the same groups of animals. NLX-112 (befiradol or F13640; 3-chloro-4-fluorophenyl- [4-fluoro-4-([(5-methylpyridin-2-yl)methylamino]methyl) piperidin-1-yl]methanone, fumarate salt), NLX-101 (F15599; (3-Chloro-4-fluorophenyl-(4-fluoro-4-{[(5-methylpyrimidin-2-ylmethyl)-amino]-methyl}-piperidin-1-yl)-methanone, fumarate salt)) and F13714 (3-chloro-4-fluorophenyl-(4-fluoro-4-{[(5-methyl-6-methylaminopyridin-2-ylmethyl)-amino]-methyl}-piperidin-1-yl-methanone, fumarate salt)) and WAY-100,635 maleate (N-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]- N-(2-pyridyl) cyclohexanecarboxamide, maleate salt). NLX-101, F13714 and NLX-112 were provided by Neurolixis; WAY-100,635 was commercially obtained from Abcam (ab120550). NLX compounds and WAY-100,635 were dissolved in 0.9% (w/v) sterile sodium chloride to 0.64 mg/kg; NLX drugs were then serially diluted to 0.04 mg/kg. Clozapine-N-oxide was purchased from AK scientific (Melbourne, Australia) and dissolved in 2% dimethyl sulfoxide (DMSO) in 0.9% (w/v) sterile sodium chloride. Tandospirone hydrochloride was purchased from Tocris (Cat. No. 2854, Tocris, Australia), dissolved in 2% dimethyl sulfoxide (DMSO) in 0.9% (w/v) sterile sodium chloride and chronically injected intraperitoneally (3 mg/kg/day). For brain infusion experiments, NLX compounds (1, 16 and 32 μg) and CNO (10 and 100 μM) were dissolved in artificial cerebro-spinal fluid, aCSF (in mM: 130 NaCl, 3 KCl, 26 NaHCO3, 1.25 NaH2PO4, 5 MgCl2, 10 D-glucose). All doses refer to the weight of the free base. All the drugs were tested by an experimenter blind to the treatment on alcohol consumption by intraperitoneal (i.p.) injections (10 ml/kg, 30 min prior to presentation of the bottles) or local brain infusion (0.1 μl/min, 15 min prior presentation of the bottles) in a pseudo random Latin-square design, where each mouse received each of the three doses of the drug over the testings, with each mouse serving as its own control. Systemic active doses of NLX-101, F13714, NLX-112 and WAY-100,635 were selected based on previous studies in rodents [38, 42, 43]. Intracranial doses of F13714 were based on previous publications [38, 43, 44].
Locomotor activity
Ethanol-naïve 10-week-old mice were habituated 90 min/day, for 3 consecutive days, in the locomotor activity recording box (35 L × 35 W × 50 H cm3) and their activity video-tracked using the Any-Maze software. Day 1 was habituation to the box, Day 2 and 3 were habituation to the box with i.p. of saline solution 30 min into the start of the 90 min session. On Day 4, mice were treated with 0.64 mg/kg i.p. of the 5-HT1A receptor agonists (NLX-101, NLX-112 or F13714) or saline, and locomotor activity was recorded for 150 min post-injection. The effect of local CNO (100 μM) microinjection in the hippocampus on locomotor activity was tested similarly in long-term ethanol-exposed MR-DREADDed mice in a subgroup of n = 4 mice, previously tested for ethanol intake, following one week of drug-washout period.
Stereotaxic Surgeries
Following 4 weeks (short-term) or 10 weeks (long-term) of “drinking-in-the-dark” alcohol intake, mice underwent stereotaxic surgeries with implantation of guide cannula, microinjection of AAV-DREADD or both procedures. Guide cannula: Mice under isoflurane anaesthesia (2-5%, 1 L/min oxygen) were unilaterally (right) implanted with a guide cannula (26 gauge, PlasticOne) sitting 0.5 mm above the DRN (AP -4.5 mm, ML + 1.4 mm, DV 2.8 mm from bregma with a 25° medio-lateral angle [45]) or the MRN (AP -4.5 mm, ML + 1.25 mm, DV 4.0 mm from bregma, with a 15° medio-lateral angle), or bilaterally in the dentate gyri of the hippocampus (AP -2.0 mm, ML ± 1.5 mm, DV 1.5 mm from bregma). During 2 weeks of recovery, handling, and procedure habituation, mice continued the ethanol drinking procedure. AAV-DREADDs microinjection: CRE-dependent excitatory (AAV9-DIO-hSyn-hM3Dq-mCherry), inhibitory (AAV9-DIO-hSyn-hM4Di-mCherry) or control (AAV9-DIO-hSyn-mCherry) DREADDs constructs were purchased from Addgene (#50459, #44361, and #44362, titers ≥1 × 10¹³ vg/mL, USA). AAV vectors were microinjected in the DRN or MRN at the aforementioned coordinates. Three injections of 0.33 μl each were done along 3 different depths (1 μl total) along the dorso-ventral axis (+0.15, 0 and -0.15 mm from targeted region) at 10 nl/s using a Nanoject III (Drummond Scientific, Adelab). Three weeks after AAV-DREADD infection, CNO was administered i.p. at 1 mg/kg. Three weeks after cannulation with or without viral infection, 0.5 ul of F13714 or CNO was administered by a microinjection cannula (33 gauge) protruding 0.5 mm beyond the tip of the guide cannula, at a flow rate of 0.2 ul/min, in their home cage, with the injection cannula left in place for 5 min prior to removal. Ethanol bottles were presented in the DID 15 min after drug infusion.
Histology
Mice were transcardially perfused with a 4% (w/v) paraformaldehyde solution, their brains extracted and post-fixed overnight at 4 °C. Brains were sectioned on a vibratome (VT1200S, Leica Biosystem, Australia), 40 μm free-floating coronal sections were used for microinjection cannula placement verification by light microscopy, or for immunohistochemistry experiments. Only animals with cannula correctly placed or viral vector correctly expressed in the target brain region were included in the analysis.
Immunohistochemistry
Sections were incubated in permeabilization solution (Phosphate-buffer-saline 0.1 M, PBS; 1% TritonX-100; 0.1% Tween-20) for 1 h at room temperature, and rinsed in 2 × 5 min washes in antigen-retrieval (AR) solution (10 mM sodium citrate, 0.05%, tween-20 pH = 6.0), placed in a prewarmed AR solution at 80 °C for 30 min, cooled down to room temperature, and transferred to blocking solution (0.1 M PBS, 0.3 % Triton-X100, 0.05% Tween-20, 2% Normal Goat Serum) for 1 h at room temperature. For DREADDs infection site verification, the following primary antibodies were incubated 48 h at room temperature: mCherry (guinea pig anti-RFP 1:1000, Synaptic System #390 004), tryptophane hydroxylase 2 (Mouse anti-TPH2 antibody, Merck MAB91108, 1:500). After 3 × 5 min washes in blocking solution, corresponding secondary antibodies were incubated in blocking solution for 4 h at room temperature (goat anti-guinea pig–Alexa 594, Thermofisher # A-11076, 1:500; Goat anti-mouse biotinylated, Jackson Immunoresearch #115-065-166, 1:200). Sections were rinsed in PBST (0.1 M PBS, 0.3% Triton-X100, 0.03% Tween-20), and incubated in Streptavidin-Alexa 488 (Thermofisher, S11223, 1:1000) diluted in PBST, at room temperature for 30 min, rinsed 3 × 5 min in PBST, incubated in DAPI diluted in PBS (Thermofisher, #D1306, 1:1000), rinsed 3 × 5 min in PBS and mounted on slide with Prolong Gold antifade (Thermofisher, #P10144). For 5-HT innervation, immunohistochemistry was performed by a blind experimenter as previously described [46, 47]. Briefly, after permeabilization and blocking as above, sections were incubated with rat anti-5-HT antibody (Merck Millipore MAB352, 1:100) for 72 h at room temperature, followed by goat anti-rat biotinylated secondary antibody (Jackson Immunoresearch # 112-065-003, 1:200) for 4 h, and 30 min incubation with streptavidin-Cy3 (Thermofisher, #434315, 1:1000).
Statistical analyses
Data are expressed as the mean with SEM or individual values (DREADD experiments). GraphPad Prism 9 (Graph Pad Software Co., CA, USA) was used for all statistical analyses. Normality of the data distribution was verified using the Shapiro-Wilk test. For the locomotion experiments, the effects of the different agonists were compared to vehicle injections by measuring the area-under-curve (AUC, from 30 to 150 min post ip injection; or from 15 to 135 min post brain injection) and analysed using Student’s t-tests or one-way ANOVA. For the other experiments, comparisons between groups were analysed using one-way ANOVA for repeated measures (5-HT1A agonist testing) or two-way ANOVA for repeated measures (CNO treatment vs DREADD construct), using Geisser-Greenhouse correction for sphericity, followed, when necessary, by a Bonferroni multiple comparisons post-hoc test. A p value < 0.05 was considered significant.
Results
Systemic stimulation of Raphe nuclei 5-HT1A autoreceptors reduces ethanol consumption
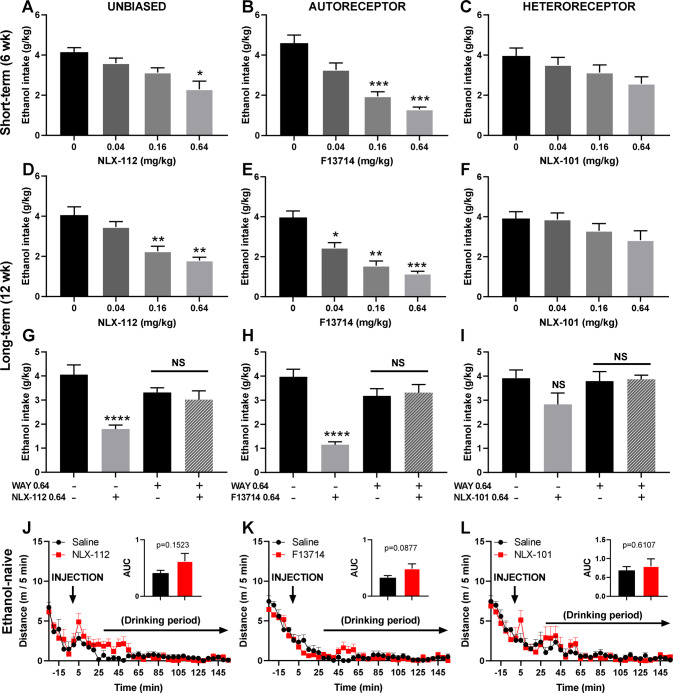
We and others have previously shown that the stimulation of 5-HT1A receptors by the agonists pindolol, buspirone or tandospirone reduces ethanol intake in mice, rats and monkeys; [27, 28, 31, 48, 49] however, due to the complex pharmacology of these drugs, with lack of selectivity and specificity at auto- vs heteroreceptors, the respective contribution of these receptor subpopulations in ethanol consumption could not be determined. Therefore, we used highly selective unbiased (NLX-112) or biased agonists targeting 5-HT1A autoreceptors (F13714) or heteroreceptors (NLX-101) to determine the respective involvement of these 5-HT1A receptor subpopulations in ethanol consumption in mice following short- (6 weeks) or long-term (12 weeks) exposure to ethanol in the “drinking-in-the dark” model. We found that administered systemically (i.p. route), the highly selective (unbiased) 5-HT1A receptor agonist NLX-112 reduces ethanol intake following short- (Fig. 1A) and long-term (Fig. 1D) exposure, as previously reported for the 5-HT1A partial agonist tandospirone [28]. We further confirmed previous observations [27] that NLX-112 showed higher potency for long- rather than short-term ethanol exposure, with a minimum effective dose (MED) of 0.16 mg/kg vs 0.64 mg/kg for long-vs short-term ethanol exposure, respectively (Fig. 1A, D). Interestingly, we found that this effect was preferentially mediated by the stimulation of 5-HT1A autoreceptors following both short- (Fig. 1B) and long-term (Fig. 1E) exposure to ethanol, with the autoreceptor-targeting agonist F13714 reducing short-term ethanol intake from 0.16 mg/kg (Fig. 1B), and long-term ethanol intake from 0.04 mg/kg (Fig. 1E). By contrast, the heteroreceptor-targeting agonist, NLX-101, did not alter ethanol intake following short- (Fig. 1C) nor long-term exposure (Fig. 1F), whatever the dose tested. Note that the absence of effects of NLX-101 is unlikely to be due to pharmacological underdosing, since NLX-101 is active at 0.16 or 0.64 mg/kg i.p. on sucrose intake in mice [42]. The specific contribution of 5-HT1A receptors in these effects was confirmed by blocking the ethanol intake-reducing effects of the highest dose (0.64 mg/kg) of NLX-112 and F13714 on long-term ethanol intake with the selective 5-HT1A receptor antagonist WAY 100,635 (0.64 mg/kg, Fig. 1G–I). The reducing effects of NLX-112 or F13714 on ethanol intake was most likely specific, since food (chow) or water consumption in food or water deprived naïve mice was unaffected at 0.64 mg/kg (supplementary Figure S1A-C). Similarly, there was no alteration in locomotor behaviour across the two-hour drinking period (Fig. 1J–L). Together these data point to somatodendritic 5-HT1A autoreceptors as playing a major role in the control of ethanol intake.
Fig. 1. Short- and long-term ethanol intake is controlled by 5-HT1A autoreceptors.
A–C Short-term ethanol intake (6 weeks) is reduced by 5-HT1A receptor stimulation, with the highest dose of the unbiased agonist NLX-112 (A, repeated-measure one-way ANOVA, n = 8, F (1.760, 12.32) = 9.049, p = 0.0047, with Bonferroni multiple comparison: *: p = 0.0114 vs vehicle). This effect is likely mediated by the activation of 5-HT1A autoreceptors as ethanol intake is dose-dependently reduced by the autoreceptor agonist F13714 (B, repeated-measure one-way ANOVA, n = 8, F(1.585, 11.10) = 20.17, p = 0.0003, with Bonferroni multiple comparison: ***: p = 0.0006 (0.16 mg/kg) and p = 0.0003 (0.64 mg/kg) vs vehicle), but not by the heteroreceptor agonist NLX-101 (C, repeated-measure one-way ANOVA, n = 8, F(2.046, 14.32) = 2.446, p = 0.1211). D-I. Long-term ethanol intake (12 weeks) is reduced by 5-HT1A receptor stimulation, with a dose-dependent effect of the unbiased agonist NLX-112 (D, repeated-measure one-way ANOVA, n = 8, F(1.945, 13.61) = 12.03, p = 0.001, with Bonferroni multiple comparison: **p = 0.0028 (0.16 mg/kg) and p = 0.0038 (0.64 mg/kg) vs vehicle). Again, this effect is likely mediated by the activation of 5-HT1A autoreceptors as ethanol intake is dose-dependently reduced by the autoreceptor agonist F13714 (E, repeated-measure one-way ANOVA, n = 7, F(2.134, 12.80) = 24.27, p < 0.0001, with Bonferroni multiple comparison: *p = 0.044, **p = 0.0051, ***p = 0.0007), but not the heteroreceptor agonist NLX-101 (F, repeated-measure one-way ANOVA, n = 8 F(1.643, 11.50) = 2.252, p = 0.15). The specific involvement of 5-HT1A receptors has been confirmed by the blockade of the effects of the highest dose of NLX-112 (G, one-way ANOVA, n = 8, F(3, 28) = 9.622, p = 0.0002, with Bonferroni multiple comparison: ****p < 0.0001 vs veh/veh control), or F13714 (H, one-way ANOVA, n = 7, F(3, 24) = 18.33, p < 0.0001, with Bonferroni multiple comparison: ****p < 0.0001 vs veh/veh control) by the selective 5-HT1A receptor antagonist WAY100635 (0.64 mg/kg). The lack of efficacy of the highest dose of NLX-101 on ethanol intake showed also no effect of the WAY100635 (n = 8, I). J–L Effects of NLX-112, F13714 and NLX-101 on locomotor activity was assessed in ethanol-naïve mice, for 2.5 h after injection, or 2 h min into the drinking session, showing no effect on locomotor activity of NLX-112 (J, t test on the area-under-curve (AUC), n = 6, p = 0.1523), F13714 (K, t test on the area-under-curve, n = 6, p = 0.0877) or NLX-101 (L, t test on the area-under-curve, n = 6, p = 0.6107).
Dorsal and median Raphe nuclei 5-HT1A autoreceptors mediate ethanol consumption following short- and long-term exposure, respectively
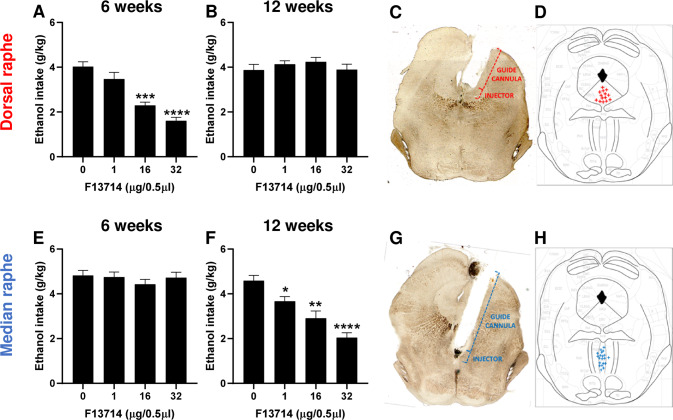
As 5-HT1A autoreceptors are found both in the DRN and MRN, where they have differential sensitivity to agonist-induced inhibition of 5-HT neuronal activity [50], we investigated the respective contribution of DRN and MRN 5-HT1A autoreceptors in ethanol intake following short- and long-term exposure, by delivering locally the autoreceptor agonist F13714 (0, 1, 16, 32 μg/0.5 μl) into either nucleus. We found that intra-DRN microinjection of F13714 dose-dependently reduced ethanol intake following short-term (6 weeks) exposure with a significant reduction at 16 and 32 μg (Fig. 2A). However, there was no effect of intra-DRN microinjection of F13714 on ethanol intake following long-term (12 weeks) exposure (Fig. 2B). Placements of microinjection cannulae were verified by histology (Fig. 2C) and only animals with correct placements were included in the analysis (Fig. 2D). As opposed to intra-DRN microinjection, we found that intra-MRN injection of F13714 had no effect on ethanol intake following short-term exposure (Fig. 2E), but dose-dependently reduced ethanol intake following long-term exposure with significant effects of all the doses (Fig. 2F). Placement of the microinjection cannulae were verified by histology (Fig. 2G) and only animals with correct placements were included in the analysis (Fig. 2H). The reducing effects of DRN and MRN microinjections of F13714 on ethanol intake was most likely specific, since food (chow) consumption in food-deprived naïve mice was unaffected by the highest dose (32 µg, supplementary Figure S1D-F). These results indicate that a switch takes place from DRN to MRN and that subpopulations of 5-HT1A autoreceptors are differentially involved in ethanol consumption between short- and long-term ethanol exposure.
Fig. 2. 5-HT1A autoreceptors in the dorsal and median raphe mediate short- and long-term ethanol intake, respectively.
A–D Local infusion of F13714 in the dorsal Raphe nucleus (DRN) dose-dependently reduces ethanol intake following short-term (6 weeks) exposure (A, repeated measure one-way ANOVA, n = 14, F(2.054, 26.70) = 34.43, p < 0.0001, with Bonferroni multiple comparison: ***p < 0.001 and ****p < 0.0001 vs vehicle) but has no effects following long-term (12 weeks exposure, (B) repeated measure one-way ANOVA, n = 14, F(2.847, 37.01) = 1.255, p = 0.3035). Cannula placement was verified by histology (C) and only animals with DRN-targeting cannulae were included (D). Local infusion of F13714 in the median Raphe nucleus (MRN) has no effect on ethanol intake following short-term (6 weeks) exposure (E, repeated measure one-way ANOVA, n = 20, F(2.784, 52.89) = 1.277, p = 0.2915) but dose-dependently- reduces ethanol intake following long-term (12 weeks) exposure (F, repeated measure one-way ANOVA, n = 20, F(2.465, 46.83) = 20.13, p < 0.0001, with Bonferroni multiple comparison: *p = 0.0376, **p = 0.0026 and ****p < 0.0001 vs vehicle). Cannula placement was verified by histology (G) and only animals with MRN-targeting cannulae were included (H).
Chemogenetic inhibition of dorsal and median Raphe nuclei 5-HT neuronal activity differentially reduces short- and long-term ethanol consumption, respectively
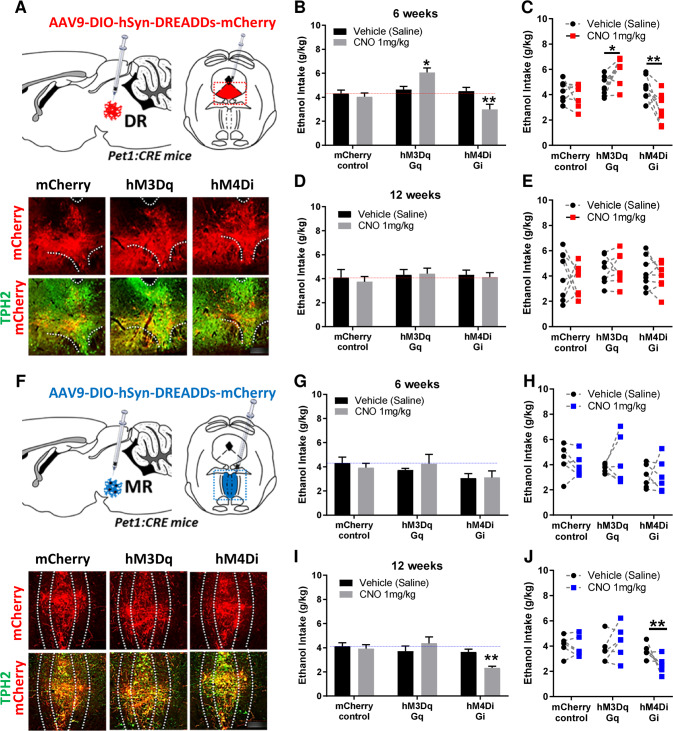
To investigate whether modulating 5-HT neuron activity in the DRN or the MRN could affect short- and long-term ethanol drinking, we used a chemogenetic approach, the Designer Receptors Exclusively Activated by Designer Drugs (DREADDs). For this, we expressed three different CRE-dependent DREADDs constructs using AAV9 serotype particles (DIO-hSyn-mCherry, DIO-hSyn-hM3Dq-mCherry and DIO-hSyn-hM4Di-mCherry) as control, excitatory and inhibitory DREADDs, respectively, in pet1 5-HT neurons (pet1-CRE mice). To control for any off-target effects of the designer drug Clozapine-N-Oxide (CNO), mice that received mCherry control constructs were also treated with the same dose of CNO (1 mg/kg i.p.). Following DRN delivery of AAV9 particles, we verified the correct expression of the 3 DREADDs constructs in TPH2 immunoreactive 5-HT neurons of the DRN (Fig. 3A, Supplementary Fig. S2). Upon stimulation of the DREADDs constructs by CNO in mice exposed to ethanol for 6 weeks, we observed a significant treatment x construct interaction effect on ethanol intake. Post-hoc multiple comparison tests revealed a significant increase in ethanol intake following stimulation of DRN 5-HT neurons and a significant decrease in ethanol intake following silencing of DRN 5-HT neurons (Fig. 3B). There was no effect of CNO in mCherry control mice when compared to mCherry control mice treated with saline. Interestingly, there was no effect of manipulating DRN 5-HT neuronal activity in the same animals that continued to consume ethanol for a total of 12 weeks (treatment x construct interaction effect) (Fig. 3D). Next, following MRN delivery of AAV9 particles we verified the correct expression of the 3 DREADDs constructs in TPH2 immunoreactive 5-HT neurons of the MRN (Fig. 3F, Supplementary Fig. S3). Upon stimulation of the DREADDs constructs by CNO (1 mg/kg) in mice exposed to ethanol for 6 weeks, there was no effect of manipulating MRN 5-HT neuron activity on ethanol consumption (treatment x construct) (Fig. 3G). However, we observed a significant treatment x construct interaction effect on ethanol intake when MRN neuron activity was modulated in long-term ethanol consuming mice (12 weeks) (Fig. 3I). Multiple comparison post-hoc tests revealed a significant decrease in ethanol intake following silencing of MR 5-HT neurons. The effects of chemogenetic modulation of 5-HT neuron activity are likely specific to ethanol consumption as stimulating or inhibiting DRN (6 weeks) or MRN (12 weeks) had no effects on sucrose consumption (supplementary Figure S1G-J). These results provide further evidence that 5-HT neuron activity in the DRN plays a role in short-term ethanol intake, and that it switches to MRN following long-term ethanol intake.
Fig. 3. Chemogenetic modulations of dorsal and median Raphe 5-HT neuron activity differentially affect short and long-term ethanol consumption.
A–E mCherry-control, hM3Dq-excitatory and hM4Di-inhibitory DREADDs were injected in pet1-5-HT DRN neurons and their expression in TPH2-immunoreactive neurons was confirmed by immunohistochemistry (A, micrograph field corresponding to the red dashed square in the diagram above, scale bar: 150 µm). Manipulation of DREADD-expressing neurons in the DRN by systemic CNO (1 mg/kg) bidirectionally modulated short-term (6 weeks) ethanol intake, with their stimulation increasing ethanol intake, and their inhibition reducing ethanol intake (B–C, repeated measure two-way ANOVA, n = 8, treatment x construct: F (2, 21) = 11.34; p = 0.0005; with Bonferroni multiple comparison: *p = 0.0113, **p = 0.0072). However, this modulation of ethanol intake by DRN 5-HT neurons was lost long-term exposure to ethanol (D–E, repeated measure two-way ANOVA, n = 8, treatment x construct: F (2, 21) = 0.1371; p = 0.8727). mCherry-control, hM3Dq-excitatory and hM4Di-inhibitory DREADDs were then injected in pet1-5-HT MRN neurons and their expression in TPH2-immunoreactive neurons was confirmed by immunohistochemistry (F, micrograph field corresponding to the blue dashed square in the diagram above, scale bar: 100 µm). Manipulation of DREADD-expressing neurons in the MR by intraperitoneal CNO (1 mg/kg) had no effect on short-term (6 weeks) ethanol intake (G–H, repeated measure two-way ANOVA, n = 6, treatment x construct: F (2, 15) = 0.5862, p = 0.5687). However, inhibition of MRN 5-HT neurons by systemic CNO (1 mg/kg) reduced long-term ethanol intake (I–J, repeated measure two-way ANOVA, n = 6, treatment x construct: F (2, 15) = 9.318, p = 0.0023, with Bonferroni multiple comparison: **p = 0.0031), while their activation had no effect (I-J, p = 0.1836).
Long-term consumption of ethanol alters 5-HT innervation in the DG in a 5-HT1A receptor-dependent manner
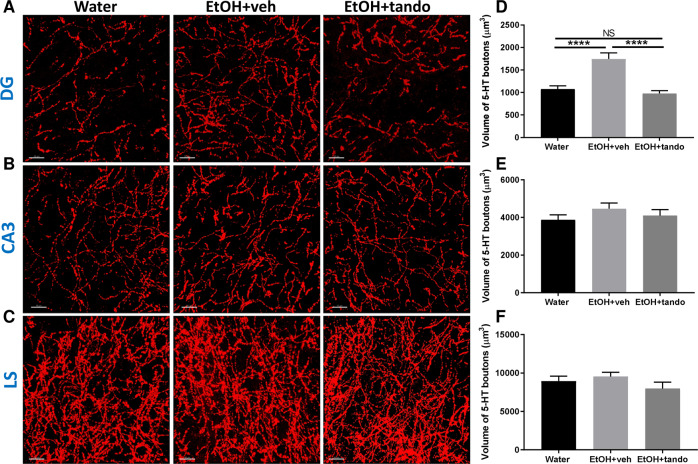
We observed above that 5-HT1A autoreceptors from the MRN control ethanol intake under long-term exposure conditions. The 5-HT innervation ascending from the MRN projects densely to the dentate gyrus (DG), the CA3 region of the hippocampus and the lateral septum (LS) [51–53]. We therefore investigated the effect of long-term ethanol consumption on 5-HT innervation of these projection brain regions. Following 12 weeks of ethanol consumption, 5-HT-immunoreactive axons were labelled, 3D-reconstructed (Fig. 4A–C) and quantified (Fig. 4D–F) as previously described [46, 47, 54, 55]. The volume of 5-HT varicosities (boutons) in the DG, CA3 and LS was quantified in water-exposed animals (Water), ethanol-exposed animals chronically treated (2 weeks, following 12 weeks of consumption) with either vehicle (EtOH + veh) or with the 5-HT1A receptor partial agonist, tandospirone at 3 mg/kg/day, i.p. (EtOH + tando) (Fig. 4D–F). We found that long-term ethanol consumption significantly increases the volume of 5-HT-immunoreactive boutons in the DG (Fig. 4D) but not in the CA3 (Fig. 4E) or LS (Fig. 4F). Interestingly chronic treatment with the 5-HT1A agonist tandospirone prevented this increase in the DG and restored the volume of 5-HT varicosities to the level of water-control animals (Fig. 4D) while having no effect on 5-HT innervation in the CA3 or LS. This suggests that long-term consumption of ethanol produces activity-dependent alterations in the morphology of 5-HTMRN>DG neurons that likely contribute to the maintenance of long-term ethanol intake.
Fig. 4. Long-term ethanol exposure alters 5-HT neurons innervation in the DG and is reversed by chronic 5-HT1A agonist treatment.
5-HT-immunoreactive axons (red) from the dentate gyrus (DG; A), the CA3 region of the hippocampus (CA3; B) and lateral septum (LS; C) of mice exposed to water (water; left panel), ethanol—treated with vehicle (EtOH + veh, middle panel) or ethanol—chronically treated with the 5-HT1A agonist tandospirone (EtOH + tando; right panel) were labelled and the varicosities reconstructed in 3D. Scale bar: 15 μm. Total volume of reconstructed varicosities of mice exposed to water (water; black), ethanol - treated with vehicle (EtOH + veh, light grey) or ethanol - chronically treated with the 5-HT1A agonist tandospirone (EtOH + tando; dark grey) was quantified in the DG (D), CA3 (E) and LS (F). Mean volume ± SEM (in μm3). One-way ANOVA, n = 6, ****p < 0.0001, NS non-significant, p > 0.9999.
5-HTMRN→DG neuronal circuit activity modulates long-term ethanol intake
To determine the role played by 5-HTMRN>DG neurons in the maintenance of long-term ethanol drinking behaviour, we used chemogenetics (AAV-DIOhSyn-DREADDs-mCherry constructs) in the MRN, combined with local microinjections of the designer drug CNO in the DG to investigate the effect of specific stimulation (hM3Dq construct variant) or silencing (hM4Di construct variant) of the activity of this neuronal circuit on ethanol intake following long-term exposure (Fig. 5A). The correct expression of the viral construct in the MRN was verified by immunohistochemistry (Fig. 5B) and the correct placements of guide-cannulae in the DG were verified by histology (Fig. 5C). We observed that the stimulation of 5-HTMRN→DG neurons by the highest concentration of CNO (100 μM or 20 ng/0.5 µl) significantly increases, while silencing of 5-HTMRN→DG neurons significantly decreases ethanol intake, during the initial binge-phase of ethanol drinking sessions (first 30 min of the 2 h session) (Fig. 5D–E). The initial increase in ethanol intake elicited by 5-HTMRN→DG neurons activation by CNO (100 μM) did not persist over the entire drinking session whereas inhibition of 5-HTMRN→DG neurons dose-dependently reduced ethanol intake for the whole 2 h (Fig. 5F–G). We then assessed whether these alterations in drinking behaviour were mediated by changes in locomotor activity after DG injection of CNO (100 μM). We found that the stimulation of 5-HTMRN>DG neurons by hM3Dq-CNO or silencing by hM4Di-CNO did not affect overall locomotor activity compared to mCherry-control mice (Fig. 5H–I). This suggests that the reduction in ethanol intake is not resulting from altered locomotor behaviour.
Fig. 5. Chemogenetic manipulation of 5-HTMRN>DG circuit modulates ethanol intake following long-term exposure.
A–C mCherry-control, hM3Dq-excitatory, and hM4Di-inhibitory DREADDs were injected in pet1-5-HT MRN neurons and bilateral cannulae were implanted in the hippocampus, above the dentate gyrus (A). Expression of DREADD constructs in TPH2-immunoreactive MRN neurons was verified by immunohistochemistry (B, field corresponding to the red dashed square in A) and the correct cannulae placement verified by histology (C). Chemogenetic manipulation of 5-HTMRN>DG neuron terminals by local infusion of CNO (100 μM) bidirectionally modulated the binge-consumption of ethanol (first 30 min of a 2 h drinking period) (D–E, repeated measure two-way ANOVA, n = 6, treatment x construct: F(4, 30) = 6.545, p = 0.0007). Bonferroni multiple comparison showed that the stimulation of these terminals increased the 30 min intake of ethanol (D–E, **p = 0.074), while their inhibition reduced the binge-intake of ethanol (D–E, *p = 0.013). Chemogenetic manipulation of 5-HTMRN>DG neuron terminals also modulated the 2 h consumption of ethanol (F–G, repeated measure two-way ANOVA, n = 6, treatment: F(1.883, 28.25) = 3.902, p = 0.0341), with Bonferroni multiple comparison showing that only the inhibition of these terminals reduced the overall 2 h intake of ethanol (F–G *p = 0.0239; **p = 0.0076), but no effect of chemogenetic activation (p > 0.99). Effect of intra-DG injection of CNO on locomotor activity (H) showing no effect over the 2-hour drinking period (I, One-way ANOVA on AUC [15–135 min], n = 4, F (2, 9) = 0.4343, p = 0.6606).
Discussion
The present study reveals that long-term ethanol intake changes the role of 5-HT1A-autoreceptor-dependent activity in the DRN and the MRN. As the length of ethanol exposure increases, there is a switch from a DRN- to MRN-mediated control of ethanol intake. This switch is likely mediated by ethanol intake-induced changes in 5-HT innervation observed in dentate gyrus (DG) of the hippocampus, as we have shown that 5-HTMRN→DG circuits mediate long-term ethanol consumption.
Our laboratory has previously demonstrated the 5-HT1A receptor-dependent modulation of ethanol consumption, without determining the respective involvement of 5-HT1A auto- or heteroreceptors in this mechanism [27, 28, 31, 56]. The present study showed for the first time the exclusive contribution of 5-HT1A autoreceptors in ethanol intake. Systemic treatments with the 5-HT1A autoreceptor agonist F13714 showed increased efficacy to reduce ethanol intake between short- and long-term ethanol consumption, suggesting a change in 5-HT1A autoreceptor function after long-term ethanol consumption. We further demonstrated a switch in the role of DRN to MRN 5-HT over time, with DRN-5-HT1A autoreceptor stimulation reducing short-term ethanol intake drinking, and MRN-5-HT1A autoreceptor stimulation reducing long-term ethanol intake. This suggests that the two Raphe nuclei are differentially involved in ethanol intake following short- and long-term.
5-HT1A autoreceptors play an important role in the regulation of the activity of 5-HT neurons located in either the DRN and MRN [57], where they mediate various physiological function such as reward processing, anxiety-, stress-, or fear-related behaviour [20, 58–63]. Using local microinjections of the selective 5-HT1A autoreceptor biased agonist F13714, we observed a functional transition in the role of DRN to MRN in the maintenance of short- and long-term alcohol consumption, with DRN-5-HT1A autoreceptor stimulation only reducing short-term drinking, while long-term drinking was reduced by MRN-5-HT1A autoreceptor stimulation. This suggests that the two Raphe nuclei are differentially involved in alcohol drinking behaviour following short- and long-term exposure. This time-dependent recruitment of DRN and MRN 5-HT1A autoreceptors could be due to differences in their propensity to hypersensitization following acute or chronic alcohol exposure [64], or in their variable responses to agonist stimulation [64, 65]. Interestingly, a similar shift in 5-HT1A receptor function has been observed, also from DRN to MRN, in the psychostimulant responses to chronic cocaine [66]. In addition, while nicotine-withdrawal following short-term exposure (7 days) recruits DRN 5-HT neurons [67], it is likely that nicotine-withdrawal following longer exposure (6 weeks) is mostly controlled by MRN 5-HT neuron activity [68], suggesting that a neuroadaptive shift between DRN and MRN neurotransmission could mediate the transitioning from short-term consumption to long-term substance abuse. However, an opposite shift, from MRN to DRN, has also been demonstrated in the transition from moderate to compulsive cocaine intake following SERT selective knock down in the different raphe nuclei [69]. Therefore, whether the functional shift between the DRN to MRN could underly a specific mechanism by which moderate intake of ethanol evolves into chronic binge-like or compulsive consumption remains to be elucidated.
However, raphe nuclei also contain non-serotonergic cells that express the 5-HT1A receptors, such as GABA interneurons in the DRN [70] or other undefined cell types in the MRN [71]. To determine the specific involvement of serotonergic neurons, we further investigated the functional DRN to MRN switch using chemogenetics, confirming that silencing of DRN-5-HT neurons reduces ethanol intake in short-term but not long-term alcohol-exposed mice. Conversely, silencing of MRN-5-HT neurons does not affect short-term alcohol intake while it does reduce long-term consumption. 5-HT1A autoreceptor stimulation is inhibitory of 5-HT neuron activity [72], therefore, the chemogenetic data corroborates our results with the 5-HT1A autoreceptor biased agonist F13714. Moreover, these observations suggest that alcohol consumption elicits hyperactivity of 5-HT neurons in the Raphe nuclei, as previously reported [30], first initiated in the DRN following short-term consumption, and then transferred to MRN after long-term alcohol consumption.
Raphe 5-HT1A autoreceptor sensitization or upregulation has been observed in mice, rats and monkeys following chronic alcohol consumption [26, 73, 74]. In line with an increased 5-HT1A autoreceptor inhibitory function, microdialysis studies have shown that while acute/short-term ethanol intake elevates 5-HT release in various brain regions, including the hippocampus, nucleus accumbens, striatum, amygdala, prefrontal cortex, and ventral tegmental area, chronic/long-term exposure to ethanol produces a reduction of extracellular 5-HT levels and/or 5-HT turnover in these brain regions (for review see [25]). This suggests that long-term ethanol consumption is linked to an overall reduction in 5-HT neurotransmission, however, people abusing alcohol likely have an increased expression of the tryptophan hydroxylase 2 (TPH2, the rate-limiting biosynthetic enzyme for 5-HT) [75, 76], reduced expression of the monoamine oxidase A (MAO-A, the catalysis enzyme for 5-HT) [77], and increased rates of 5-HT neuronal uptake [78, 79], which rather suggests increased 5-HT levels, presumably intracellularly. For instance, inhibitors of MAO activity have been shown to increase the intracellular content of 5-HT within serotonergic axons, in the cortex, hippocampus and thalamus [80]. Our results showing increased 5-HT immunoreactive innervation within the dentate gyrus (DG) of the hippocampus support an elevation of intracellular 5-HT levels and therefore suggest that long-term ethanol consumption augments the levels of axonal 5-HT while likely reducing its extracellular levels.
Previous work from our laboratory has demonstrated that long-term ethanol consumption elicits 5-HT1A receptor-dependent deficits in anxiety-like behaviour and hippocampal neurogenesis in the DG [28], a hallmark of chronic alcohol abuse [81]. Although serotonergic drugs that ameliorate neurogenesis have shown limited clinical efficacy in the treatment of alcohol dependence, we now confirm by a local chemogenetics approach, that the 5-HTMRN→DG neuronal circuit is involved in long-term ethanol consumption, showing that its inhibition reduces ethanol intake under these conditions. There is increasing evidence indicating that the hippocampus contributes to drug-reward processes, drug-related memory formation, and drug-induced anxiety and dysphoria (for review see [82]). It is likely that neuroadaptations produced by prolonged substance abuse augment hippocampal activity, thus amplifying the responses to substances of abuse and associated cues [82]. Hence, long-term serotonergic neuroadaptations within the hippocampus, and possibly the resulting changes in neurogenesis, may contribute to relapse vulnerability [83] through enhanced drug sensitivity, enhanced drug memory, or anxiogenic stimuli. It is noteworthy that one limitation of the present study is the use of male mice only. Therefore, since alcohol drinking behaviour, anxiety, as well as the regulation of 5-HT signalling, have shown important sex differences, further work is needed to provide a detailed characterization of the gender-specific role played by DRN and MRN 5-HT neurotransmission in alcohol binge consummatory behaviour.
Conclusion
Using a dual pharmacological and chemogenetic approach, the present study reveals that DRN and MRN 5-HT neurons are differentially involved in alcohol drinking behaviour whether it results from short- or long-term exposure, and that the functional transition from DRN to MRN-mediated behaviour might represent a mechanism by which acute alcohol consumption develops into chronic binge-like drinking behaviour. Our chemogenetic data being in complete adequation with the pharmacological data, the present study further indicates that targeting Raphe nuclei 5-HT1A autoreceptors with selective and potent agonists might represent an innovative pharmacotherapeutic strategy to combat alcohol abuse. Although the 5-HT1A autoreceptor preferential agonist F13714 showed a higher potency in reducing ethanol intake, this compound cannot be developed clinically. However, the NLX-112 compound, which reduces both short- and long-term alcohol consumption is clinically ready to be tested as a promising treatment for alcohol use disorders. Moreover, the biopharmaceutical company, Neurolixis, is currently conducting a drug discovery program on 5-HT1A receptor biased agonists, with some new chemical entities showing various profiles of biased agonism. Further work with these new compounds may unravel even more efficacious therapeutic strategies for AUDs.
Supplementary information
Acknowledgements
We are grateful to the National Health and Medical Research Council of Australia for funding this study. We would like to thank Syed Aoun Ali, Ignatius Alvarez Cooper, Fatema Nasrin, and Joshua Wang for their support in animal drinking experiments and welfare. We are thankful to PACE animal facility manager Lisa Foster and her staff Rachel Smith, Miranda Sleath, and Corey Peterson for the exquisite care of our animals.
Author contributions
AB: Conceptualization, Investigation, Data curation, Formal analysis, Methodology, Validation, Writing—original draft, Writing—review & editing, Visualization, Supervision, Project administration. RD: Validation, Writing—original draft, Writing—review & editing. KB: Investigation, Data curation. AN-T: Resources, Conceptualization, Writing—review & editing, Supervision. SEB: Conceptualization, Validation, Writing—review & editing, Supervision, Project administration, Funding acquisition.
Funding
This study was supported by Equipment, Pilot Data and Seed Grants from the Queensland University of Technology to Arnauld Belmer; and by the National Health and Medical Research Council (NHMRC) (GNT1146417) and the Edge Grant from the Queensland University of Technology to Selena E Bartlett. Open Access funding enabled and organized by CAUL and its Member Institutions.
Competing interests
ANT and RD are shareholders and employees of Neurolixis and have a proprietary interest in NLX-101 and NLX-112.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Arnauld Belmer, Selena E. Bartlett.
Contributor Information
Arnauld Belmer, Email: Arnauld.belmer@qut.edu.au.
Selena E. Bartlett, Email: Selena.bartlett@qut.edu.au
Supplementary information
The online version contains supplementary material available at 10.1038/s41380-022-01789-z.
References
- 1.Wardell JD, Kempe T, Rapinda KK, Single A, Bilevicius E, Frohlich JR, et al. Drinking to cope during COVID-19 pandemic: the role of external and internal factors in coping motive pathways to alcohol use, solitary drinking, and alcohol problems. Alcohol Clin Exp Res. 2020;44:2073–83. doi: 10.1111/acer.14425. [DOI] [PubMed] [Google Scholar]
- 2.Pollard MS, Tucker JS, Green HD., Jr Changes in adult alcohol use and consequences during the COVID-19 pandemic in the US. JAMA Netw Open. 2020;3:e2022942. doi: 10.1001/jamanetworkopen.2020.22942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McPhee MD, Keough MT, Rundle S, Heath LM, Wardell JD, Hendershot CS. Depression, environmental reward, coping motives and alcohol consumption during the COVID-19 pandemic. Front Psychiatry. 2020;11:574676. doi: 10.3389/fpsyt.2020.574676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Graupensperger S, Fleming CB, Jaffe AE, Rhew IC, Patrick ME, Lee CM. Changes in young adults’ alcohol and marijuana use, norms, and motives from before to during the COVID-19 pandemic. J Adolesc Health Off Publ Soc Adolesc Med. 2021;68:658–65. doi: 10.1016/j.jadohealth.2021.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thurston A Alcohol Consumption Has Spiked During the Pandemic. Could the Consequences Outlast the Coronavirus? Boston Univ. 2021. https://www.bu.edu/articles/2021/alcohol-consumption-has-spiked-during-the-pandemic-could-the-consequences-outlast-coronavirus/. Accessed 12 Aug 2021.
- 6.Dankoski EC, Agster KL, Fox ME, Moy SS, Wightman RM. Facilitation of serotonin signaling by SSRIs is attenuated by social isolation. Neuropsychopharmacology. 2014;39:2928–37. doi: 10.1038/npp.2014.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sargin D, Oliver DK, Lambe EK. Chronic social isolation reduces 5-HT neuronal activity via upregulated SK3 calcium-activated potassium channels. ELife. 2016;5:e21416. doi: 10.7554/eLife.21416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Waider J, Popp S, Mlinar B, Montalbano A, Bonfiglio F, Aboagye B, et al. Serotonin deficiency increases context-dependent fear learning through modulation of hippocampal activity. Front Neurosci. 2019;13:245. doi: 10.3389/fnins.2019.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marcinkiewcz CA, Mazzone CM, D’Agostino G, Halladay LR, Hardaway JA, DiBerto JF, et al. Serotonin engages an anxiety and fear-promoting circuit in the extended amygdala. Nature. 2016;537:97–101. doi: 10.1038/nature19318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lowry CA, Johnson PL, Hay-Schmidt A, Mikkelsen J, Shekhar A. Modulation of anxiety circuits by serotonergic systems. Stress Amst Neth. 2005;8:233–46. doi: 10.1080/10253890500492787. [DOI] [PubMed] [Google Scholar]
- 11.Dell’Osso L, Carmassi C, Mucci F, Marazziti D. Depression, serotonin and tryptophan. Curr Pharm Des. 2016;22:949–54. doi: 10.2174/1381612822666151214104826. [DOI] [PubMed] [Google Scholar]
- 12.Ohmura Y, Tanaka KF, Tsunematsu T, Yamanaka A, Yoshioka M. Optogenetic activation of serotonergic neurons enhances anxiety-like behaviour in mice. Int J Neuropsychopharmacol. 2014;17:1777–83. doi: 10.1017/S1461145714000637. [DOI] [PubMed] [Google Scholar]
- 13.Ohmura Y, Tsutsui-Kimura I, Sasamori H, Nebuka M, Nishitani N, Tanaka KF, et al. Different roles of distinct serotonergic pathways in anxiety-like behavior, antidepressant-like, and anti-impulsive effects. Neuropharmacology. 2020;167:107703. doi: 10.1016/j.neuropharm.2019.107703. [DOI] [PubMed] [Google Scholar]
- 14.Urban DJ, Zhu H, Marcinkiewcz CA, Michaelides M, Oshibuchi H, Rhea D, et al. Elucidation of the behavioral program and neuronal network encoded by dorsal raphe serotonergic neurons. Neuropsychopharmacology. 2016;41:1404–15. doi: 10.1038/npp.2015.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teissier A, Chemiakine A, Inbar B, Bagchi S, Ray RS, Palmiter RD, et al. Activity of raphé serotonergic neurons controls emotional behaviors. Cell Rep. 2015;13:1965–76. doi: 10.1016/j.celrep.2015.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li A, Li R, Ouyang P, Li H, Wang S, Zhang X, et al. Dorsal raphe serotonergic neurons promote arousal from isoflurane anesthesia. CNS Neurosci Ther. 2021;27:941–50. doi: 10.1111/cns.13656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hasegawa E, Maejima T, Yoshida T, Masseck OA, Herlitze S, Yoshioka M, et al. Serotonin neurons in the dorsal raphe mediate the anticataplectic action of orexin neurons by reducing amygdala activity. Proc Natl Acad Sci. 2017;114:E3526–E3535. doi: 10.1073/pnas.1614552114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oikonomou G, Altermatt M, Zhang R, Coughlin GM, Montz C, Gradinaru V, et al. The serotonergic raphe promote sleep in zebrafish and mice. Neuron. 2019;103:686–701.e8. doi: 10.1016/j.neuron.2019.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Venner A, Broadhurst RY, Sohn LT, Todd WD, Fuller PM Selective activation of serotoninergic dorsal raphe neurons facilitates sleep through anxiolysis. Sleep. 2020;43. [DOI] [PMC free article] [PubMed]
- 20.You I-J, Wright SR, Garcia-Garcia AL, Tapper AR, Gardner PD, Koob GF, et al. 5-HT1A Autoreceptors in the Dorsal Raphe Nucleus Convey Vulnerability to Compulsive Cocaine Seeking. Neuropsychopharmacology. 2016;41:1210–22. doi: 10.1038/npp.2015.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Browne CJ, Abela AR, Chu D, Li Z, Ji X, Lambe EK, et al. Dorsal raphe serotonin neurons inhibit operant responding for reward via inputs to the ventral tegmental area but not the nucleus accumbens: evidence from studies combining optogenetic stimulation and serotonin reuptake inhibition. Neuropsychopharmacology. 2019;44:793–804. doi: 10.1038/s41386-018-0271-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sengupta A, Holmes A. A discrete dorsal raphe to basal amygdala 5-HT circuit calibrates aversive memory. Neuron. 2019;103:489–505.e7. doi: 10.1016/j.neuron.2019.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Szőnyi A, Zichó K, Barth AM, Gönczi RT, Schlingloff D, Török B, et al. Median raphe controls acquisition of negative experience in the mouse. Science 2019;366. [DOI] [PubMed]
- 24.Balázsfi D, Zelena D, Demeter K, Miskolczi C, Varga ZK, Nagyváradi Á, et al. Differential roles of the two raphe nuclei in amiable social behavior and aggression – an optogenetic study. Front Behav Neurosci. 2018;12:163. doi: 10.3389/fnbeh.2018.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Belmer A, Patkar OL, Pitman KM, Bartlett SE. Serotonergic neuroplasticity in alcohol addiction. Brain Plast. 2016;1:177–206. doi: 10.3233/BPL-150022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kelaï S, Renoir T, Chouchana L, Saurini F, Hanoun N, Hamon M, et al. Chronic voluntary ethanol intake hypersensitizes 5-HT(1A) autoreceptors in C57BL/6J mice. J Neurochem. 2008;107:1660–70. doi: 10.1111/j.1471-4159.2008.05733.x. [DOI] [PubMed] [Google Scholar]
- 27.Patkar OL, Belmer A, Holgate JY, Tarren JR, Shariff MR, Morgan M, et al. The antihypertensive drug pindolol attenuates long-term but not short-term binge-like ethanol consumption in mice. Addict Biol. 2017;22:679–91. doi: 10.1111/adb.12359. [DOI] [PubMed] [Google Scholar]
- 28.Belmer A, Patkar OL, Lanoue V, Bartlett SE. 5-HT1A receptor-dependent modulation of emotional and neurogenic deficits elicited by prolonged consumption of alcohol. Sci Rep. 2018;8:2099. doi: 10.1038/s41598-018-20504-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Underwood MD, Mann JJ, Arango V. Morphometry of dorsal raphe nucleus serotonergic neurons in alcoholism. Alcohol Clin Exp Res. 2007;31:837–45. doi: 10.1111/j.1530-0277.2007.00365.x. [DOI] [PubMed] [Google Scholar]
- 30.Lowery-Gionta EG, Marcinkiewcz CA, Kash TL. Functional alterations in the dorsal raphe nucleus following acute and chronic ethanol exposure. Neuropsychopharmacology. 2015;40:590–600. doi: 10.1038/npp.2014.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patkar OL, Belmer A, Beecher K, Jacques A, Bartlett SE. Pindolol rescues anxiety-like behavior and neurogenic maladaptations of long-term binge alcohol intake in mice. Front Behav Neurosci. 2019;13:264. doi: 10.3389/fnbeh.2019.00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sniecikowska J, Newman-Tancredi A, Kolaczkowski M. From receptor selectivity to functional selectivity: the rise of biased agonism in 5-HT1A receptor drug discovery. Curr Top Med Chem. 2019;19:2393–420. doi: 10.2174/1568026619666190911122040. [DOI] [PubMed] [Google Scholar]
- 33.Becker G, Bolbos R, Costes N, Redouté J, Newman-Tancredi A, Zimmer L. Selective serotonin 5-HT1A receptor biased agonists elicitdistinct brain activation patterns: a pharmacoMRI study. Sci Rep. 2016;6. [DOI] [PMC free article] [PubMed]
- 34.Depoortere R, Bardin L, Auclair AL, Slot LAB, Newman-Tancredi A. Marble burying in NMRI male mice is preferentially sensitive to pre- versus postsynaptic 5-HT1A receptor biased agonists. Pharmacology. 2021;106:114–8. doi: 10.1159/000509729. [DOI] [PubMed] [Google Scholar]
- 35.Mannoury la Cour C, El Mestikawy S, Hanoun N, Hamon M, Lanfumey L. Regional differences in the coupling of 5-hydroxytryptamine-1A receptors to G proteins in the rat brain. Mol Pharm. 2006;70:1013–21. doi: 10.1124/mol.106.022756. [DOI] [PubMed] [Google Scholar]
- 36.Newman-Tancredi A, Martel J-C, Cosi C, Heusler P, Lestienne F, Varney MA, et al. Distinctive in vitro signal transduction profile of NLX-112, a potent and efficacious serotonin 5-HT1A receptor agonist. J Pharm Pharm. 2017;69:1178–90. doi: 10.1111/jphp.12762. [DOI] [PubMed] [Google Scholar]
- 37.Newman-Tancredi A, Martel J-C, Assié M-B, Buritova J, Lauressergues E, Cosi C, et al. Signal transduction and functional selectivity of F15599, a preferential post-synaptic 5-HT1A receptor agonist. Br J Pharm. 2009;156:338–53. doi: 10.1111/j.1476-5381.2008.00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Newman-Tancredi A, Depoortère RY, Kleven MS, Kołaczkowski M, Zimmer L. Translating biased agonists from molecules to medications: Serotonin 5-HT1A receptor functional selectivity for CNS disorders. Pharm Ther. 2022;229:107937. doi: 10.1016/j.pharmthera.2021.107937. [DOI] [PubMed] [Google Scholar]
- 39.Scott MM, Wylie CJ, Lerch JK, Murphy R, Lobur K, Herlitze S, et al. A genetic approach to access serotonin neurons for in vivo and in vitro studies. Proc Natl Acad Sci USA. 2005;102:16472–7. doi: 10.1073/pnas.0504510102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Belmer A, Quentin E, Diaz SL, Guiard BP, Fernandez SP, Doly S, et al. Positive regulation of raphe serotonin neurons by serotonin 2B receptors. Neuropsychopharmacology. 2018;43:1623–32. doi: 10.1038/s41386-018-0013-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav. 2005;84:53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 42.Beecher K, Wang J, Chehrehasa F, Depoortere R, Varney MA, Newman-Tancredi A, et al. Dissecting the contribution of 5-HT1A auto- and heteroreceptors in sucrose overconsumption in mice. Biomed Pharmacother. 2022;148:112699. doi: 10.1016/j.biopha.2022.112699. [DOI] [PubMed] [Google Scholar]
- 43.Depoortère R, Auclair AL, Newman-Tancredi A. NLX-101, a highly selective 5-HT1A receptor biased agonist, mediates antidepressant-like activity in rats via prefrontal cortex 5-HT1A receptors. Behav Brain Res. 2021;401:113082. doi: 10.1016/j.bbr.2020.113082. [DOI] [PubMed] [Google Scholar]
- 44.Newman-Tancredi A, Bardin L, Auclair A, Colpaert F, Depoortère R, Varney MA. NLX-112, a highly selective 5-HT1A receptor agonist, mediates analgesia and antidepressant-like activity in rats via spinal cord and prefrontal cortex 5-HT1A receptors, respectively. Brain Res. 2018;1688:1–7. doi: 10.1016/j.brainres.2018.03.016. [DOI] [PubMed] [Google Scholar]
- 45.Paxinos and Franklin’s the Mouse Brain in Stereotaxic Coordinates, Compact - 5th Edition. https://www.elsevier.com/books/paxinos-and-franklins-the-mouse-brain-in-stereotaxic-coordinates-compact/franklin/978-0-12-816159-3. Accessed 14 March 2022.
- 46.Beecher K, Wang J, Jacques A, Chaaya N, Chehrehasa F, Belmer A, et al. Sucrose consumption alters serotonin/glutamate co-localisation within the prefrontal cortex and hippocampus of mice. Front Mol Neurosci. 2021;14:133. doi: 10.3389/fnmol.2021.678267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Belmer A, Beecher K, Jacques A, Patkar OL, Sicherre F, Bartlett SE. Axonal non-segregation of the vesicular glutamate transporter VGLUT3 within serotonergic projections in the mouse forebrain. Front Cell Neurosci. 2019;13:193. doi: 10.3389/fncel.2019.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hedlund L, Wahlström G. Buspirone as an inhibitor of voluntary ethanol intake in male rats. Alcohol Alcohol Oxf Oxfs. 1996;31:149–56. doi: 10.1093/oxfordjournals.alcalc.a008126. [DOI] [PubMed] [Google Scholar]
- 49.Collins DM, Myers RD. Buspirone attenuates volitional alcohol intake in the chronically drinking monkey. Alcohol. 1987;4:49–56. doi: 10.1016/0741-8329(87)90060-7. [DOI] [PubMed] [Google Scholar]
- 50.Blier P, Serrano A, Scatton B. Differential responsiveness of the rat dorsal and median raphe 5-HT systems to 5-HT1 receptor agonists and p-chloroamphetamine. Synap N. Y N. 1990;5:120–33. doi: 10.1002/syn.890050206. [DOI] [PubMed] [Google Scholar]
- 51.Muzerelle A, Scotto-Lomassese S, Bernard JF, Soiza-Reilly M, Gaspar P. Conditional anterograde tracing reveals distinct targeting of individual serotonin cell groups (B5–B9) to the forebrain and brainstem. Brain Struct Funct. 2016;221:535–61. doi: 10.1007/s00429-014-0924-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vertes RP, Fortin WJ, Crane AM. Projections of the median raphe nucleus in the rat. J Comp Neurol. 1999;407:555–82. [PubMed] [Google Scholar]
- 53.McKenna JT, Vertes RP. Collateral projections from the median raphe nucleus to the medial septum and hippocampus. Brain Res Bull. 2001;54:619–30. doi: 10.1016/s0361-9230(01)00465-8. [DOI] [PubMed] [Google Scholar]
- 54.Belmer A, Klenowski PM, Patkar OL, Bartlett SE. Mapping the connectivity of serotonin transporter immunoreactive axons to excitatory and inhibitory neurochemical synapses in the mouse limbic brain. Brain Struct Funct. 2017;222:1297–314. doi: 10.1007/s00429-016-1278-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tarren JR, Lester HA, Belmer A, Bartlett SE. Acute ethanol administration upregulates synaptic α4-subunit of neuronal nicotinic acetylcholine receptors within the nucleus accumbens and amygdala. Front Mol Neurosci. 2017;10:338. doi: 10.3389/fnmol.2017.00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Patkar OL, Belmer A, Holgate JY, Klenowski PM, Bartlett SE. Modulation of serotonin and noradrenaline in the BLA by pindolol reduces long-term ethanol intake. Addict Biol. 2019;24:652–63. doi: 10.1111/adb.12630. [DOI] [PubMed] [Google Scholar]
- 57.Judge SJ, Gartside SE. Firing of 5-HT neurones in the dorsal and median raphe nucleus in vitro shows differential alpha1-adrenoceptor and 5-HT1A receptor modulation. Neurochem Int. 2006;48:100–7. doi: 10.1016/j.neuint.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 58.Nakamura K. The role of the dorsal raphé nucleus in reward-seeking behavior. Front Integr Neurosci. 2013;7:60. doi: 10.3389/fnint.2013.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Luo M, Zhou J, Liu Z. Reward processing by the dorsal raphe nucleus: 5-HT and beyond. Learn Mem. 2015;22:452–60. doi: 10.1101/lm.037317.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McDevitt RA, Neumaier JF. Regulation of dorsal raphe nucleus function by serotonin autoreceptors: a behavioral perspective. J Chem Neuroanat. 2011;41:234–46. doi: 10.1016/j.jchemneu.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Higgins GA, Jones BJ, Oakley NR. Effect of 5-HT1A receptor agonists in two models of anxiety after dorsal raphe injection. Psychopharmacol (Berl) 1992;106:261–7. doi: 10.1007/BF02801982. [DOI] [PubMed] [Google Scholar]
- 62.Andrews N, Hogg S, Gonzalez LE, File SE. 5-HT1A receptors in the median raphe nucleus and dorsal hippocampus may mediate anxiolytic and anxiogenic behaviours respectively. Eur J Pharm. 1994;264:259–64. doi: 10.1016/0014-2999(94)00473-0. [DOI] [PubMed] [Google Scholar]
- 63.Avanzi V, Brandão ML. Activation of somatodendritic 5-HT1A autoreceptors in the median raphe nucleus disrupts the contextual conditioning in rats. Behav Brain Res. 2001;126:175–84. doi: 10.1016/s0166-4328(01)00254-6. [DOI] [PubMed] [Google Scholar]
- 64.Beck SG, Pan Y-Z, Akanwa AC, Kirby LG. Median and dorsal raphe neurons are not electrophysiologically identical. J Neurophysiol. 2004;91:994–1005. doi: 10.1152/jn.00744.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kreiss DS, Lucki I. Chronic administration of the 5-HT1A receptor agonist 8-OH-DPAT differentially desensitizes 5-HT1A autoreceptors of the dorsal and median raphe nuclei. Synapse. 1997;25:107–16. doi: 10.1002/(SICI)1098-2396(199702)25:2<107::AID-SYN1>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 66.Szumlinski KK, Frys KA, Kalivas PW. Dissociable roles for the dorsal and median raphé in the facilitatory effect of 5-HT1A receptor stimulation upon cocaine-induced locomotion and sensitization. Neuropsychopharmacology. 2004;29:1675–87. doi: 10.1038/sj.npp.1300473. [DOI] [PubMed] [Google Scholar]
- 67.Sperling R, Commons KG. Shifting topographic activation and 5-HT1A receptor-mediated inhibition of dorsal raphe serotonin neurons produced by nicotine exposure and withdrawal. Eur J Neurosci. 2011;33:1866–75. doi: 10.1111/j.1460-9568.2011.07677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nishitani N, Ohmura Y, Kobayashi K, Murashita T, Yoshida T, Yoshioka M. Serotonin neurons in the median raphe nucleus bidirectionally regulate somatic signs of nicotine withdrawal in mice. Biochem Biophys Res Commun. 2021;562:62–68. doi: 10.1016/j.bbrc.2021.05.052. [DOI] [PubMed] [Google Scholar]
- 69.Verheij MMM, Contet C, Karel P, Latour J, van der Doelen RHA, Geenen B, et al. Median and dorsal raphe serotonergic neurons control moderate versus compulsive cocaine intake. Biol Psychiatry. 2018;83:1024–35. doi: 10.1016/j.biopsych.2017.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Marinelli S, Schnell SA, Hack SP, Christie MJ, Wessendorf MW, Vaughan CW. Serotonergic and nonserotonergic dorsal raphe neurons are pharmacologically and electrophysiologically heterogeneous. J Neurophysiol. 2004;92:3532–7. doi: 10.1152/jn.00437.2004. [DOI] [PubMed] [Google Scholar]
- 71.Bonnavion P, Bernard J-F, Hamon M, Adrien J, Fabre V. Heterogeneous distribution of the serotonin 5-HT(1A) receptor mRNA in chemically identified neurons of the mouse rostral brainstem: Implications for the role of serotonin in the regulation of wakefulness and REM sleep. J Comp Neurol. 2010;518:2744–70. doi: 10.1002/cne.22331. [DOI] [PubMed] [Google Scholar]
- 72.Andrade R, Huereca D, Lyons JG, Andrade EM, McGregor KM. 5-HT1A receptor-mediated autoinhibition and the control of serotonergic cell firing. ACS Chem Neurosci. 2015;6:1110–5. doi: 10.1021/acschemneuro.5b00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nevo I, Langlois X, Laporte A-M, Kleven M, Koek W, Lima L, et al. Chronic alcoholization alters the expression of 5-HT1A and 5-HT1B receptor subtypes in rat brain. Eur J Pharm. 1995;281:229–39. doi: 10.1016/0014-2999(95)00238-g. [DOI] [PubMed] [Google Scholar]
- 74.Hillmer AT, Wooten DW, Tudorascu DL, Barnhart TE, Ahlers EO, Resch LM, et al. The effects of chronic alcohol self-administration on serotonin-1A receptor binding in nonhuman primates. Drug Alcohol Depend. 2014;144:119–26. doi: 10.1016/j.drugalcdep.2014.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bach H, Arango V, Kassir SA, Tsaava T, Dwork AJ, Mann JJ, et al. Alcoholics have more tryptophan hydroxylase 2 mRNA and protein in the dorsal and median raphe nuclei. Alcohol Clin Exp Res. 2014;38:1894–901. doi: 10.1111/acer.12414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bonkale WL, Turecki G, Austin MC. Increased tryptophan hydroxylase immunoreactivity in the dorsal raphe nucleus of alcohol-dependent, depressed suicide subjects is restricted to the dorsal subnucleus. Synap NYN. 2006;60:81–85. doi: 10.1002/syn.20278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cervera-Juanes R, Wilhem LJ, Park B, Lee R, Locke J, Helms C, et al. MAOA expression predicts vulnerability for alcohol use. Mol Psychiatry. 2016;21:472–9. doi: 10.1038/mp.2015.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Daoust M, Lhuintre JP, Ernouf D, Legrand E, Breton P, Boucly P. Ethanol intake and 3H-serotonin uptake. II: A study in alcoholic patients using platelets 3H-paroxetine binding. Life Sci. 1991;48:1977–83. doi: 10.1016/0024-3205(91)90231-y. [DOI] [PubMed] [Google Scholar]
- 79.Faraj BA, Olkowski ZL, Jackson RT. Prevalence of high serotonin uptake in lymphocytes of abstinent alcoholics. Biochem Pharm. 1997;53:53–57. doi: 10.1016/s0006-2952(96)00726-5. [DOI] [PubMed] [Google Scholar]
- 80.Nielsen K, Brask D, Knudsen GM, Aznar S. Immunodetection of the serotonin transporter protein is a more valid marker for serotonergic fibers than serotonin. Synapse. 2006;59:270–6. doi: 10.1002/syn.20240. [DOI] [PubMed] [Google Scholar]
- 81.Staples MC, Mandyam CD Thinking after drinking: impaired hippocampal-dependent cognition in human alcoholics and animal models of alcohol dependence. Front Psychiatry. 2016;7. [DOI] [PMC free article] [PubMed]
- 82.Barr JL, Bray B, Foster GL The Hippocampus as a neural link between negative affect and vulnerability for psychostimulant relapse. Hippocampus - Plast Funct. 2018. 2018. 10.5772/intechopen.70854.
- 83.Mandyam CD, Koob GF. The addicted brain craves new neurons: putative role for adult-born progenitors in promoting recovery. Trends Neurosci. 2012;35:250–60. doi: 10.1016/j.tins.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.