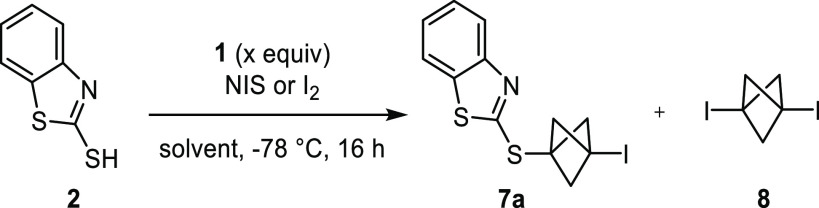
Table 1. Optimization of the Reaction Conditionsa,b.
| run | xc | iodination reagent | solvent | yield of 7a (%) | yield of 8 (%) |
|---|---|---|---|---|---|
| 1 | 1.5 | NIS (1.5 equiv) | acetone | 80 | 28 |
| 2 | 1.5 | NIS (1.5 equiv) | CH2Cl2 | 77 | 11 |
| 3 | 1.5 | NIS (1.5 equiv) | EtOAc | 80 | 18 |
| 4 | 1.5 | NIS (1.5 equiv) | toluene | 0 | 0 |
| 5 | 1.5 | NIS (1.5 equiv) | Et2O | 98 | 10 |
| 6 | 1.5 | NIS (1.5 equiv) | MTBE | 99 | 12 |
| 7 | 1.5 | NIS (1.1 equiv) | MTBE | 99 | 7 |
| 8 | 1.1 | NIS (1.1 equiv) | MTBE | 99 | 2 |
| 9 | 1.1 | NIS (1.0 equiv) | MTBE | 99 | 2 |
| 10 | 1.5 | I2 (0.75 equiv) | MTBE | 36 | 42 |
Reactions conducted with 0.2 mmol of 2 (0.2 M) and using a 0.85–1.10 M solution of 1 in Et2O.
Yields determined by 1H NMR with CH2Cl2 as the internal standard. MTBE denotes methyl tert-butyl ether.
Number of equivalents of 1.